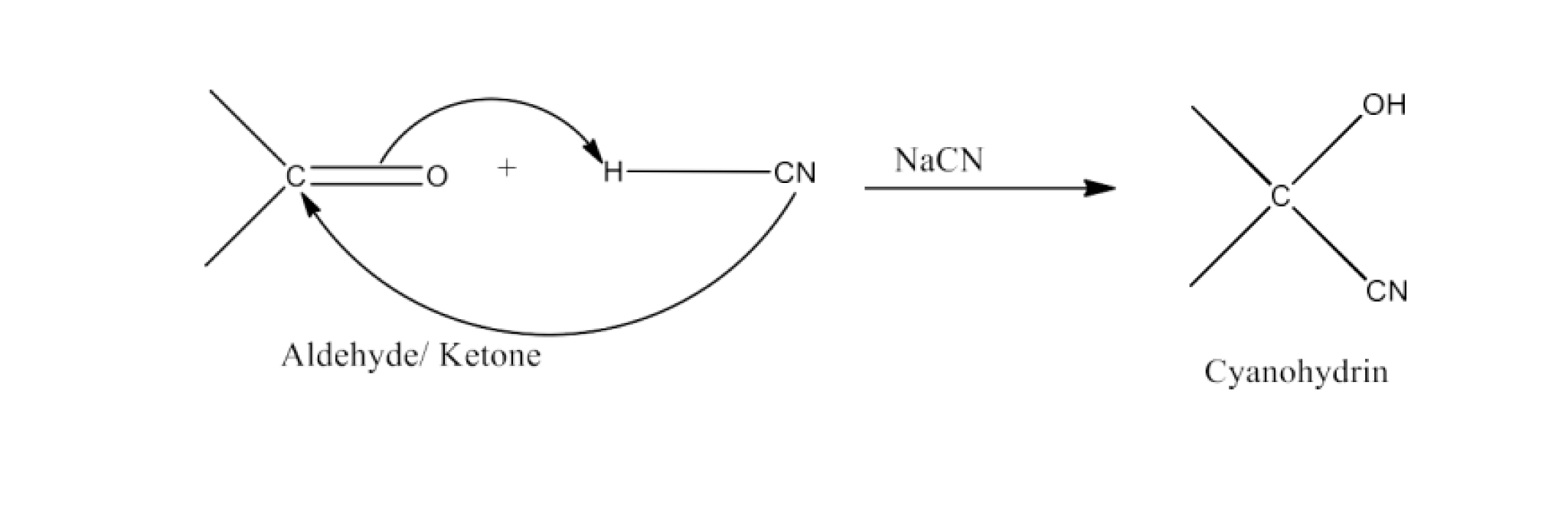
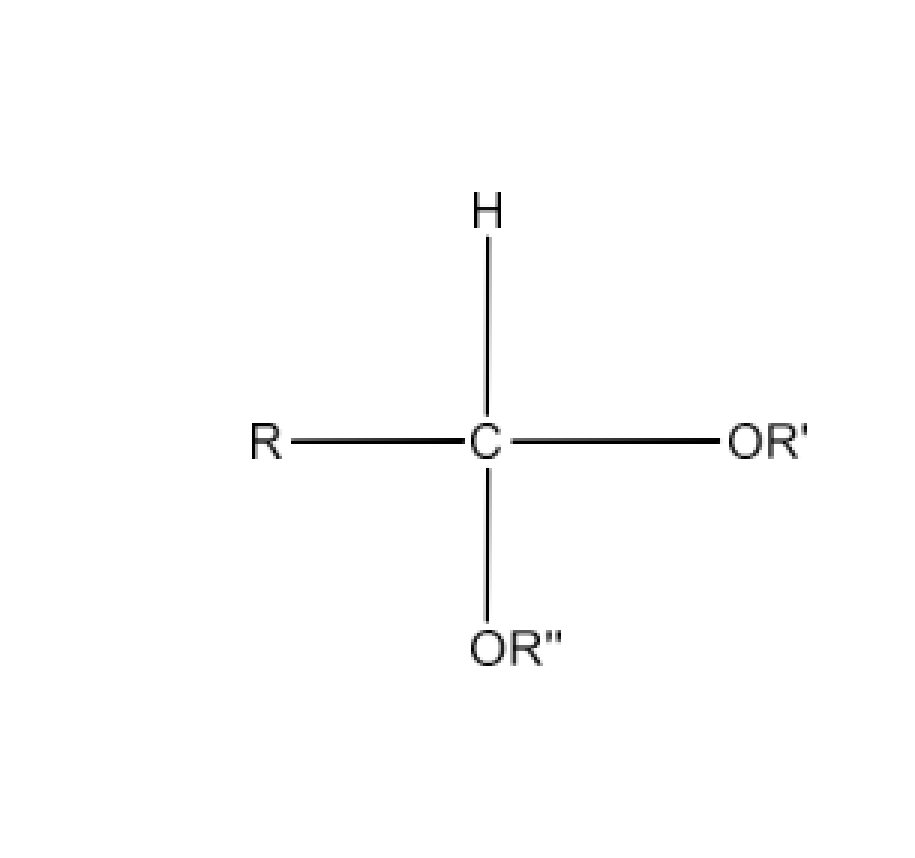
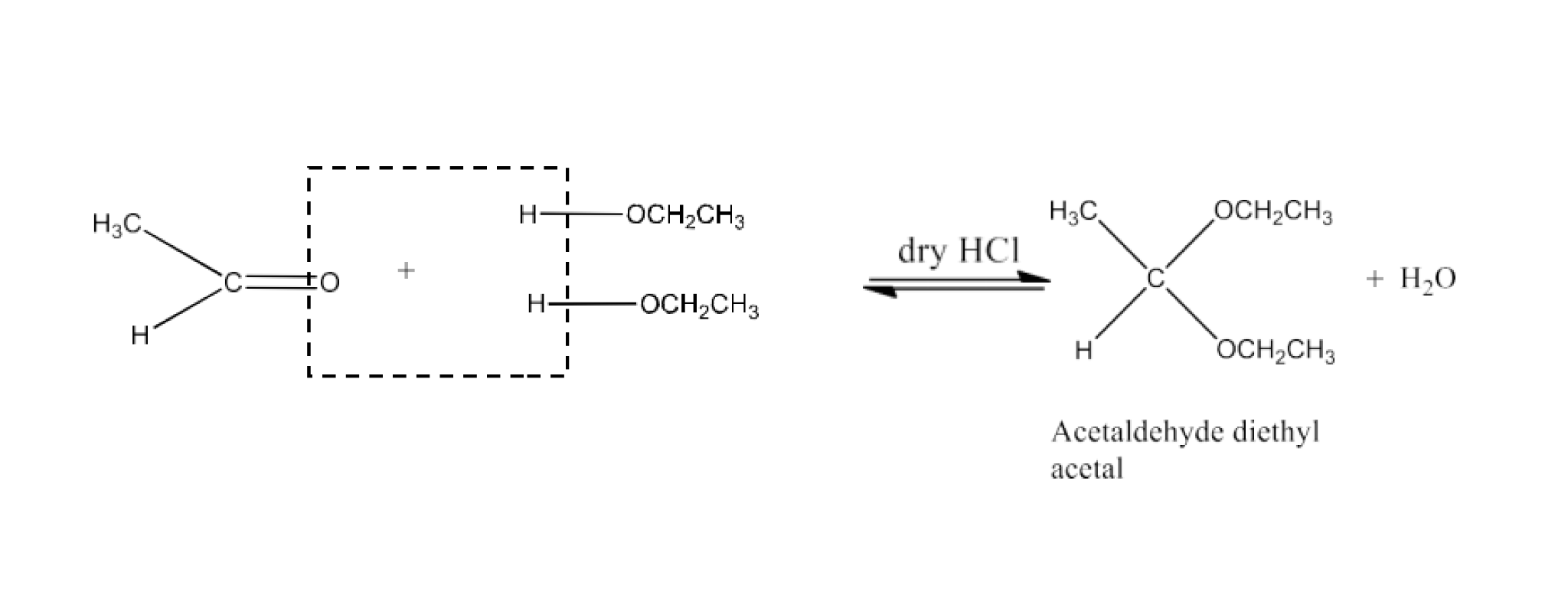
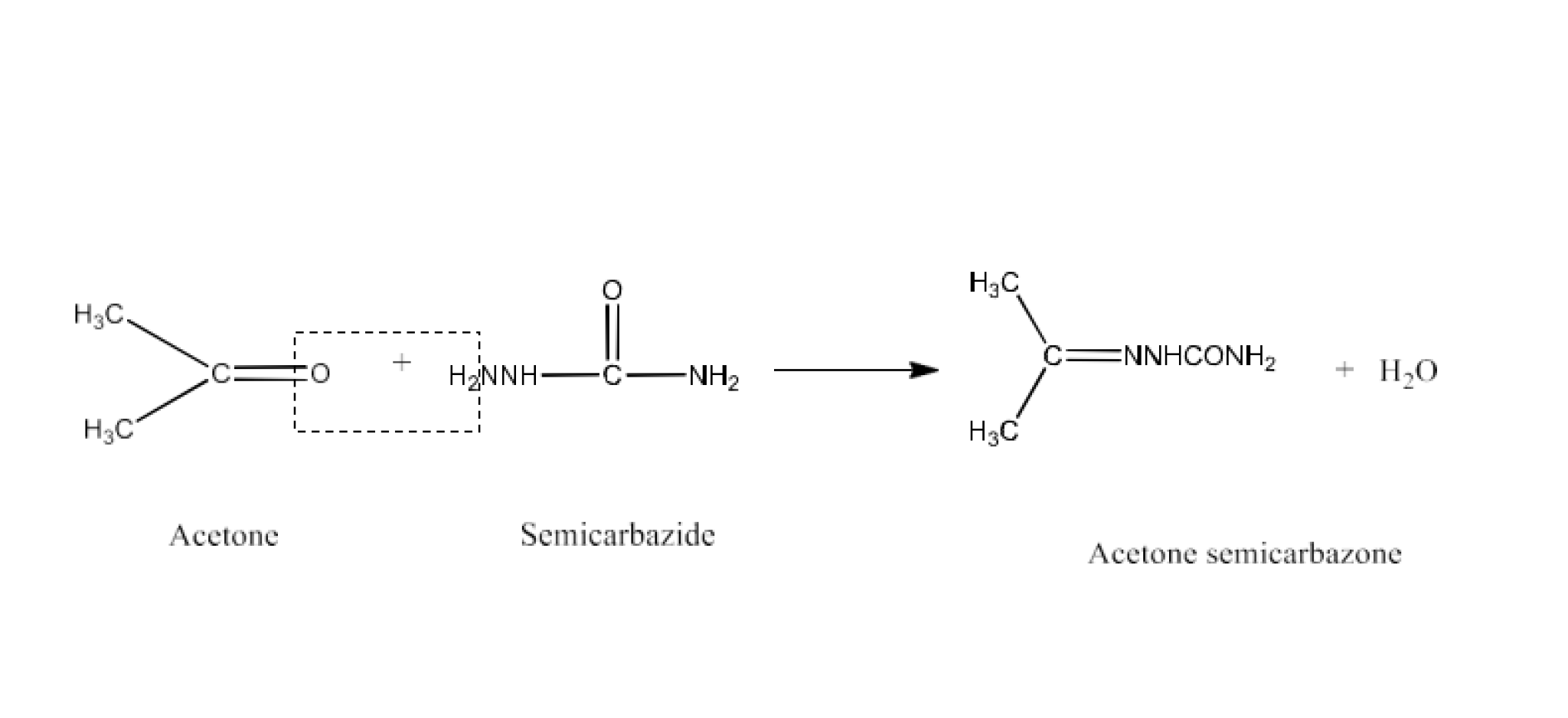
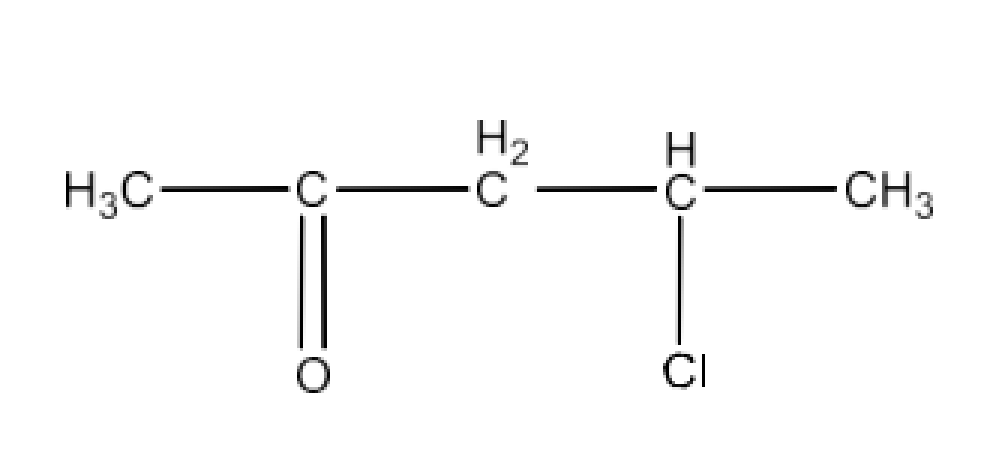
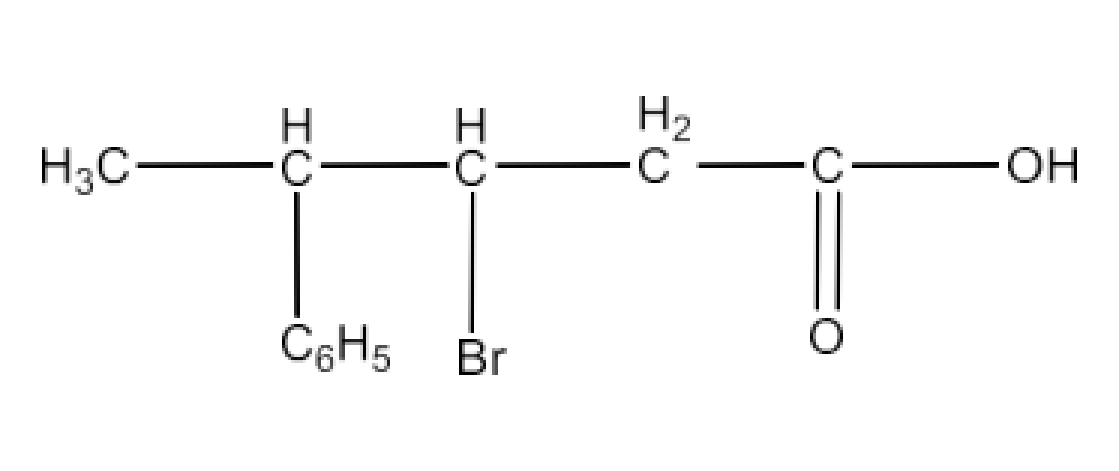
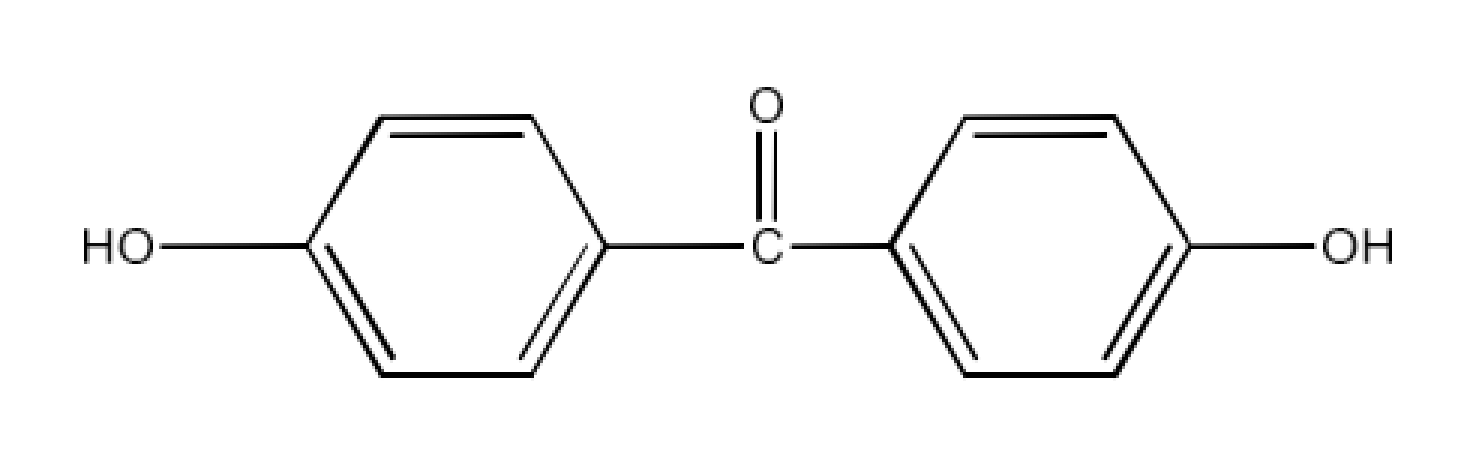
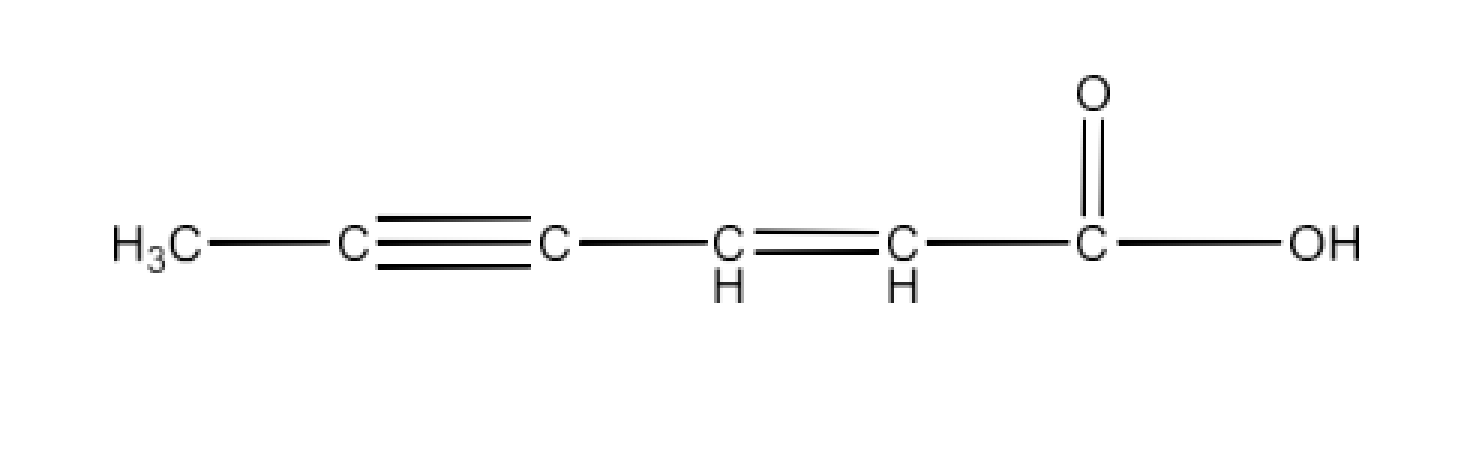
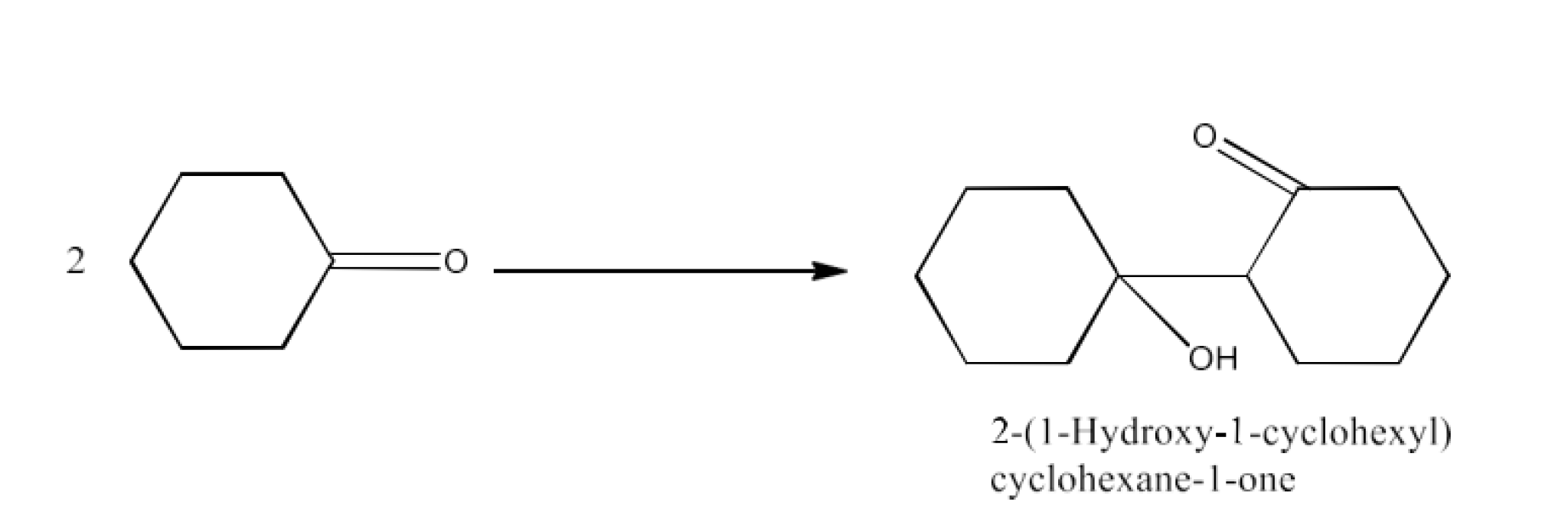
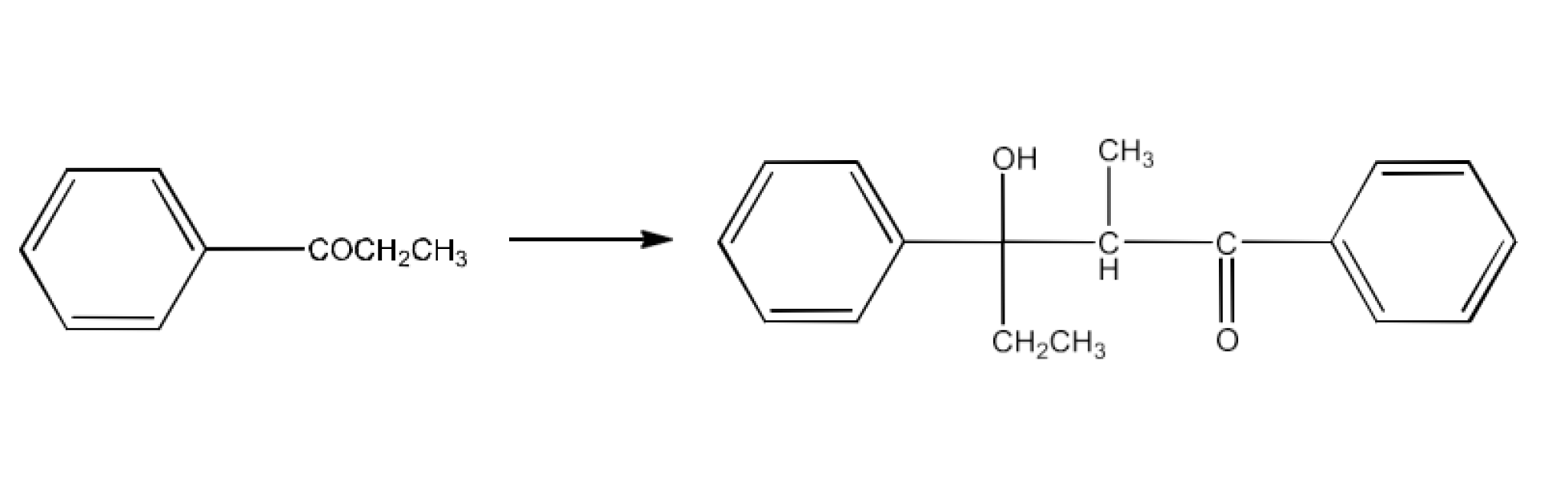
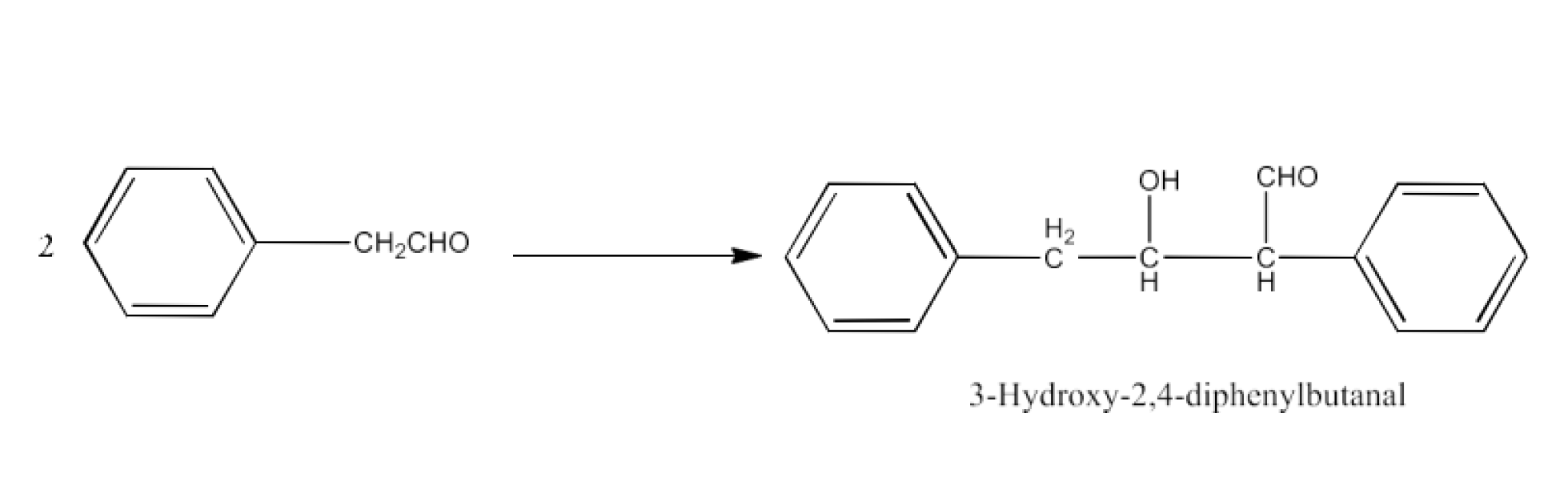
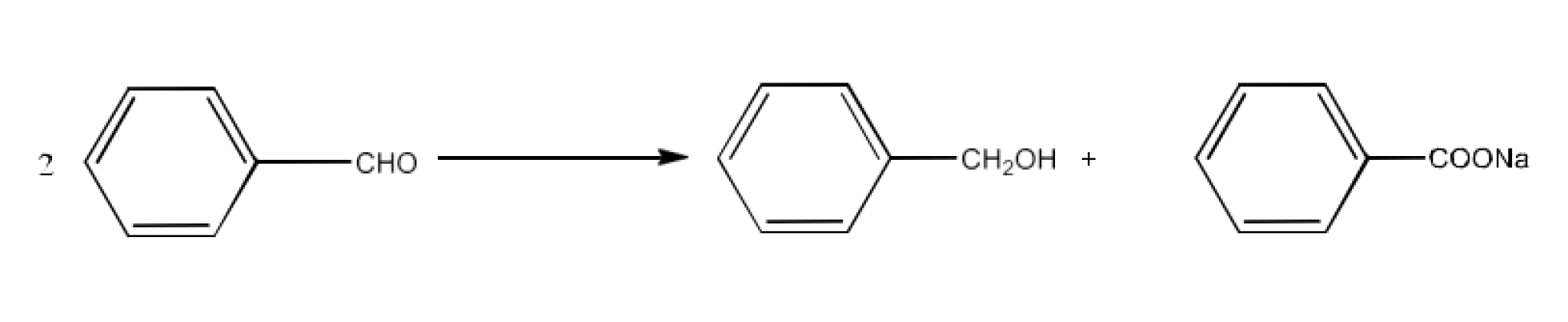
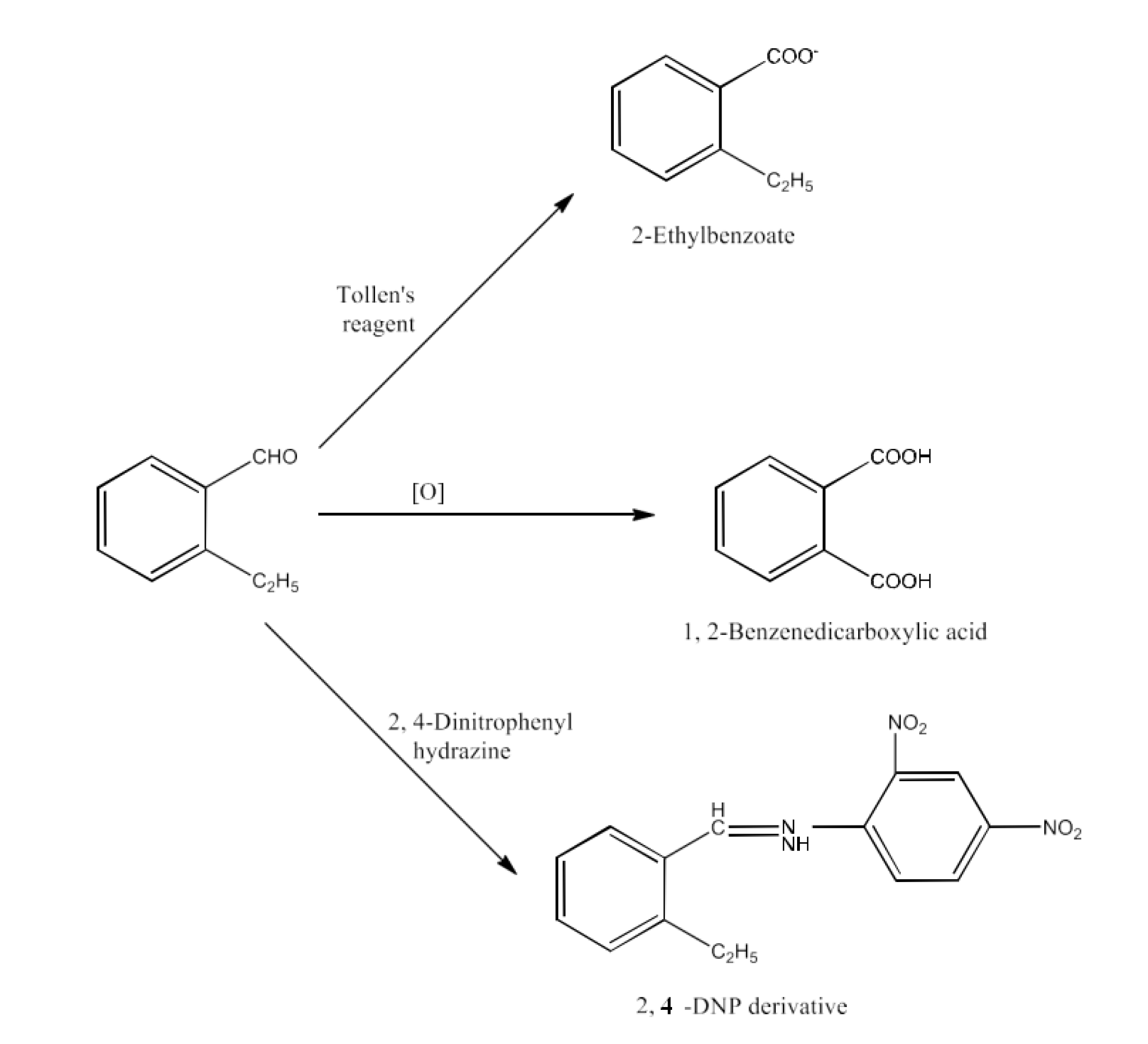
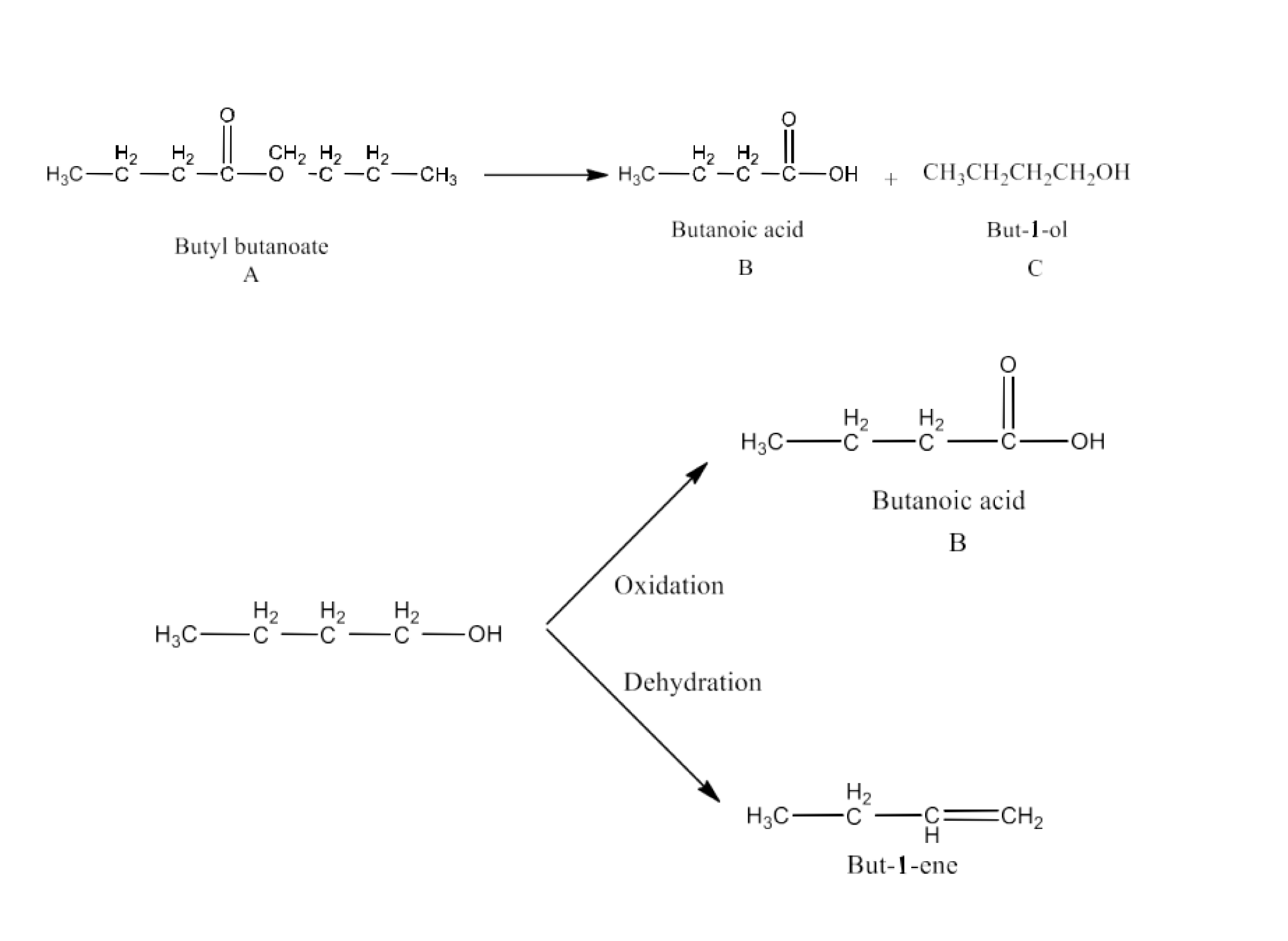
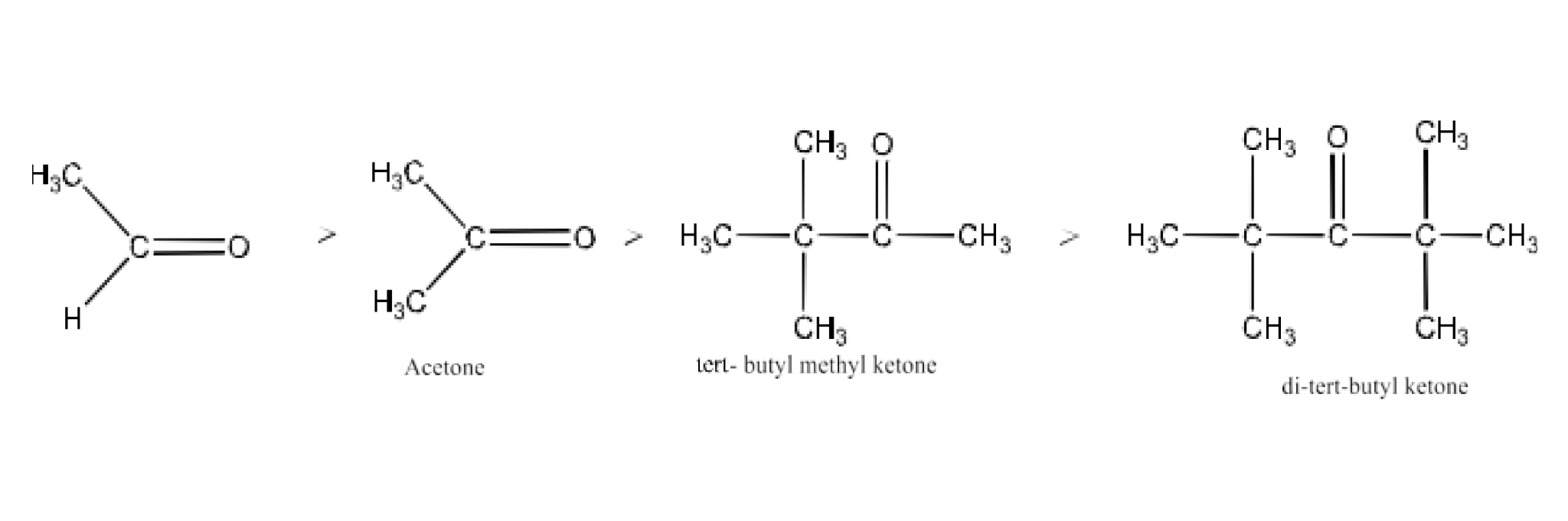
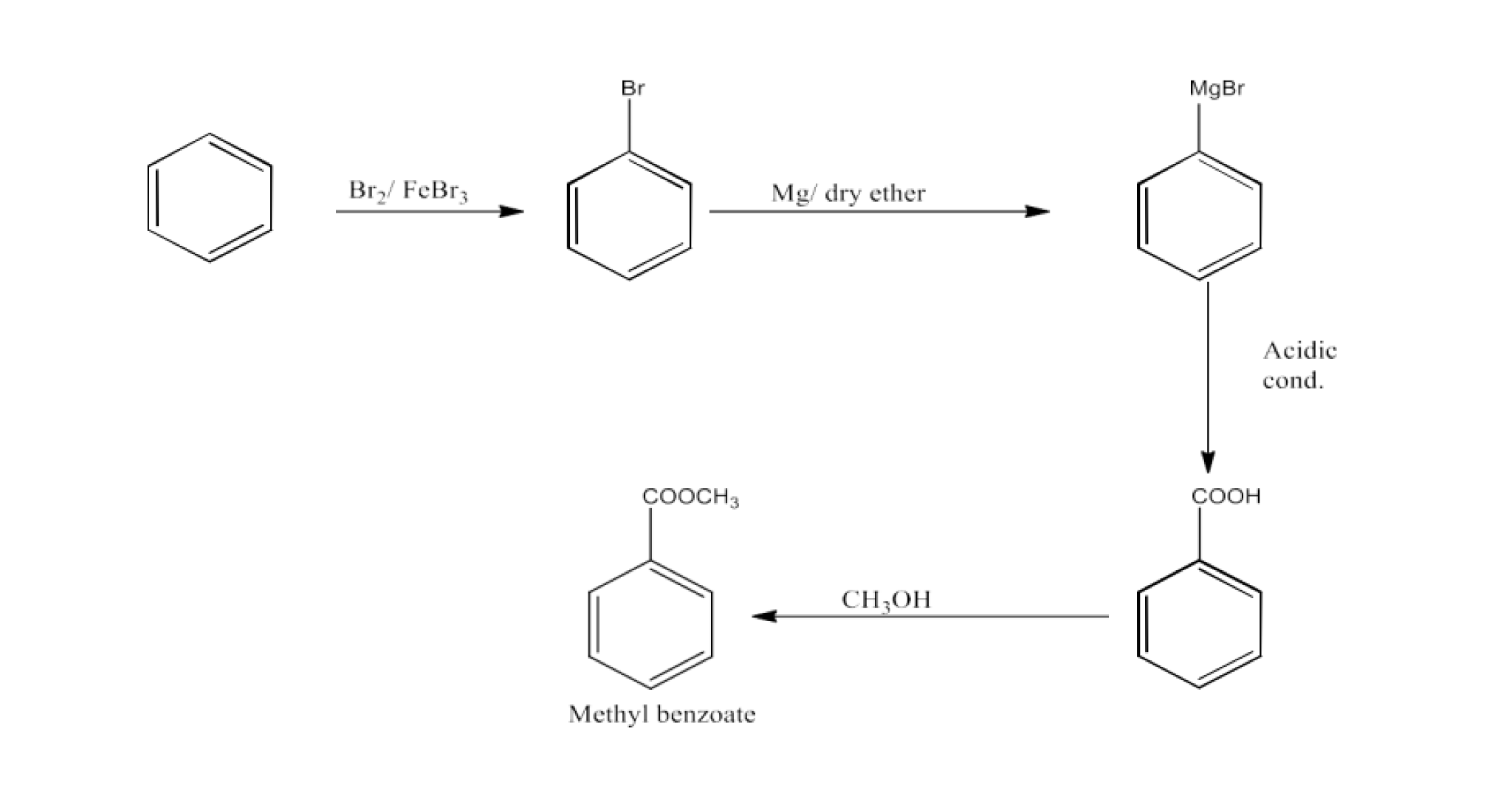
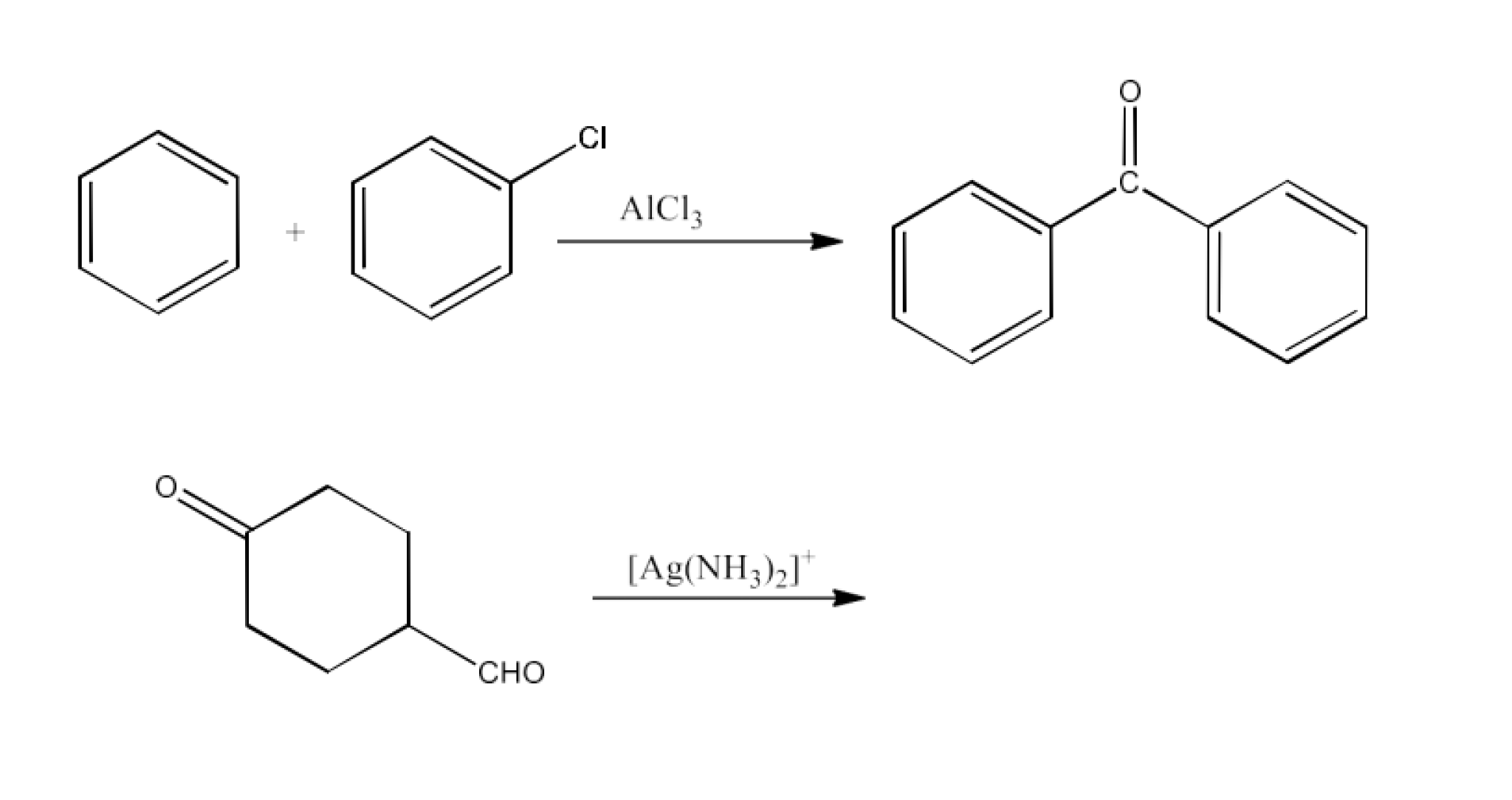
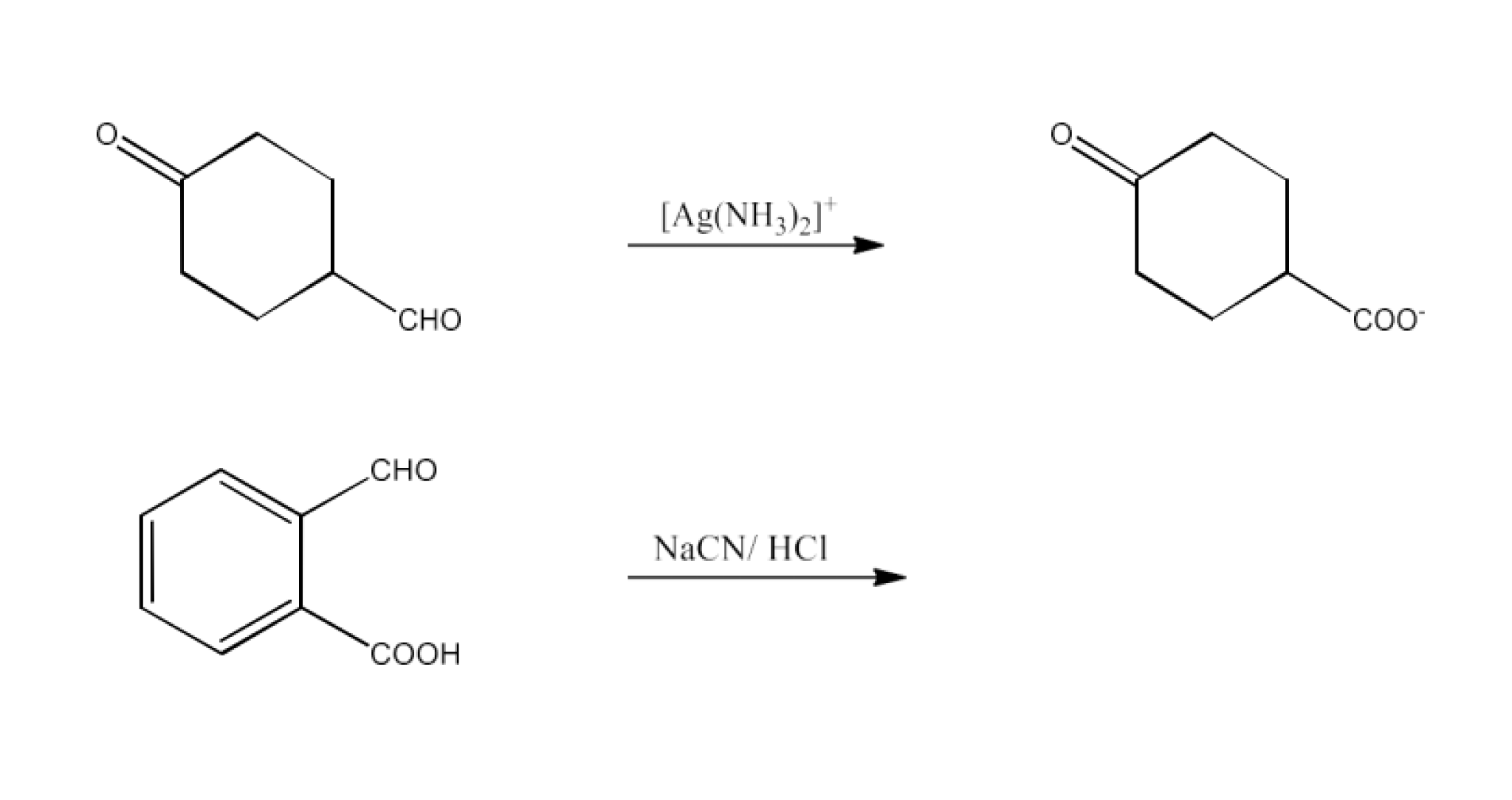
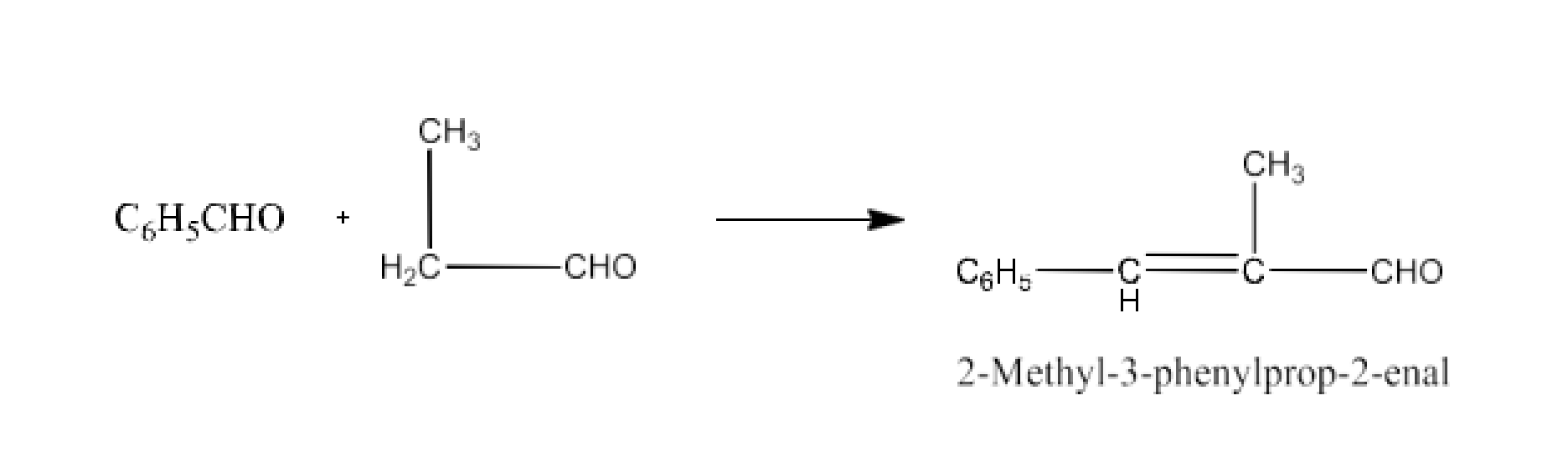
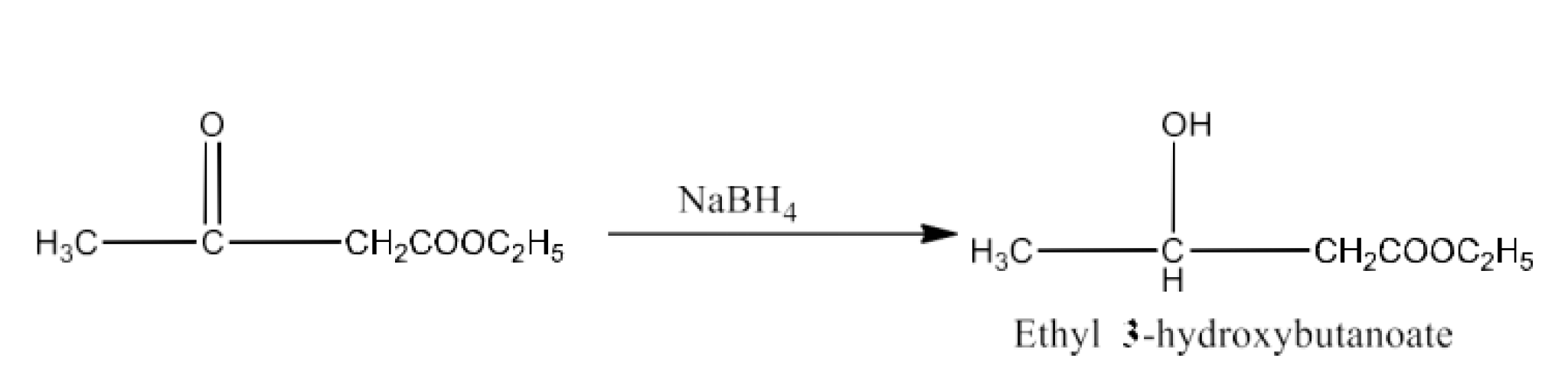
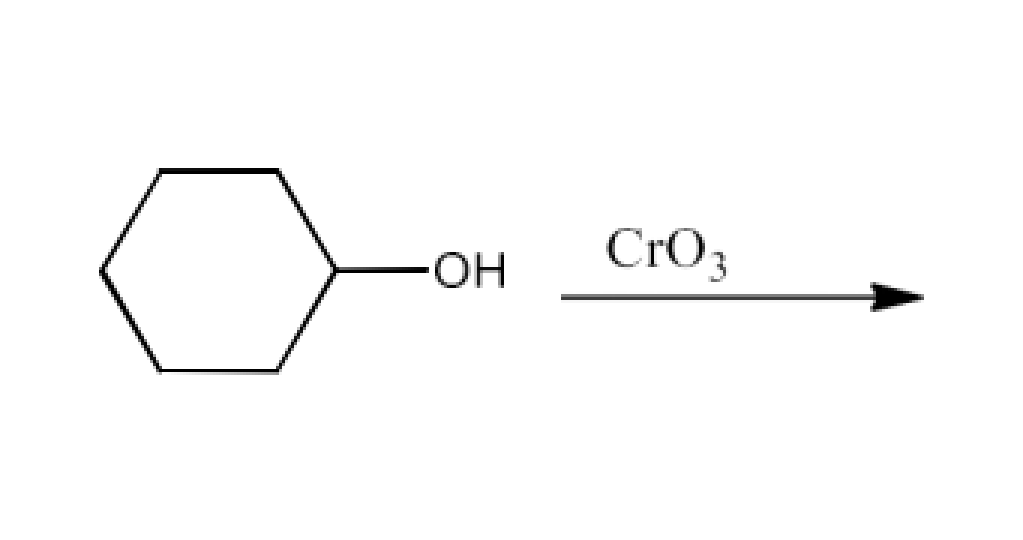
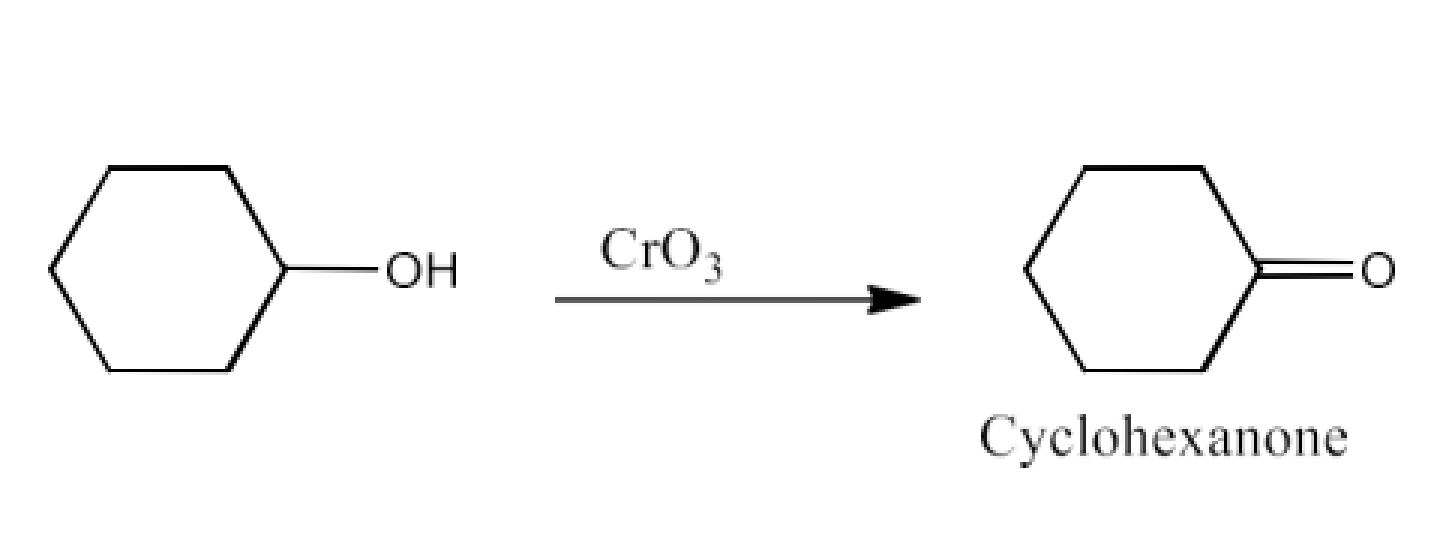
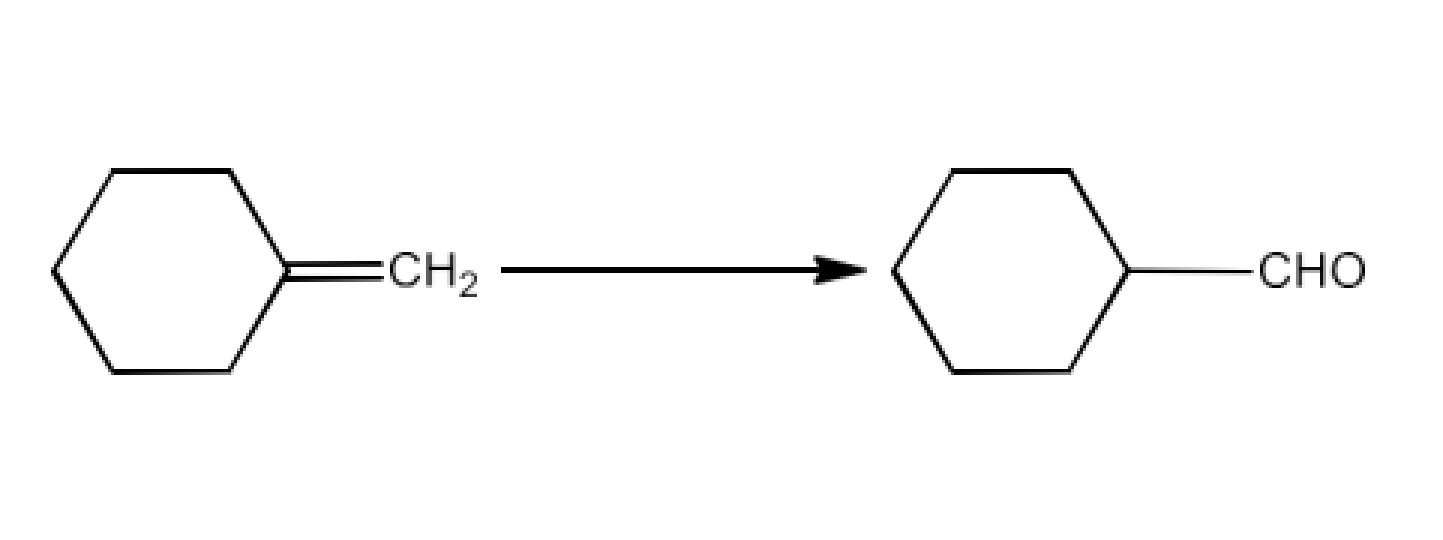
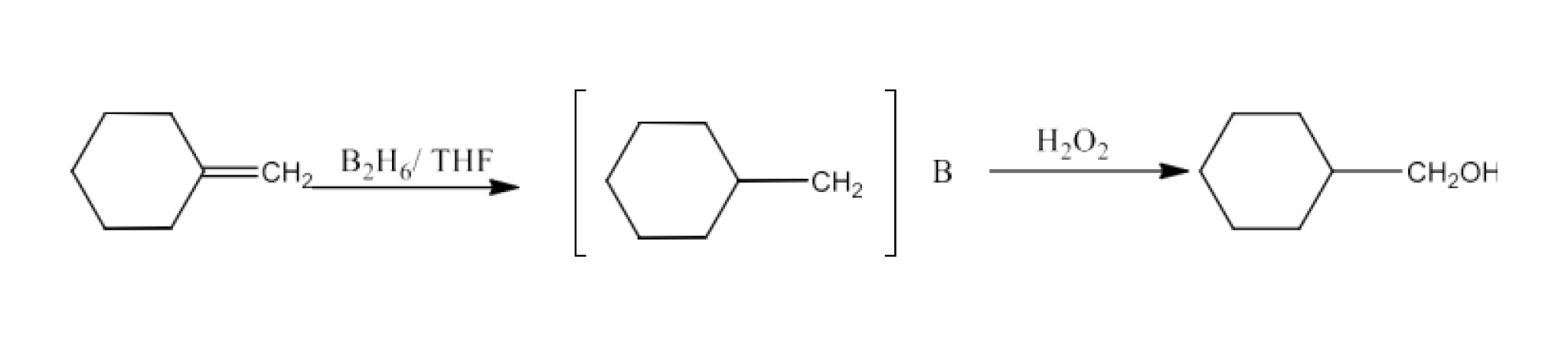
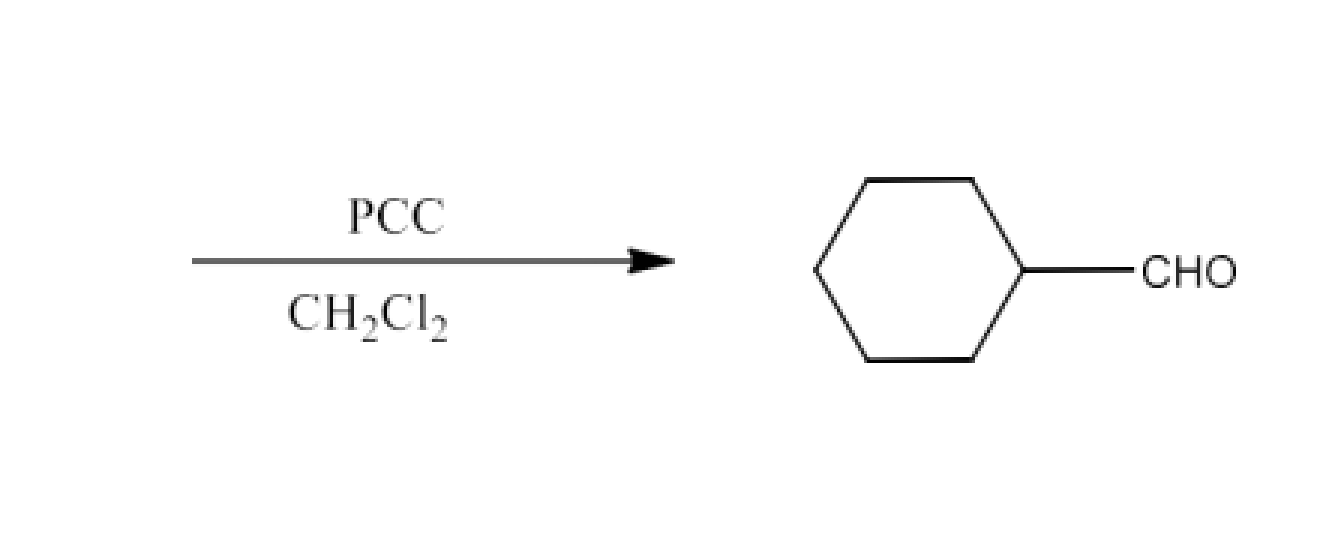
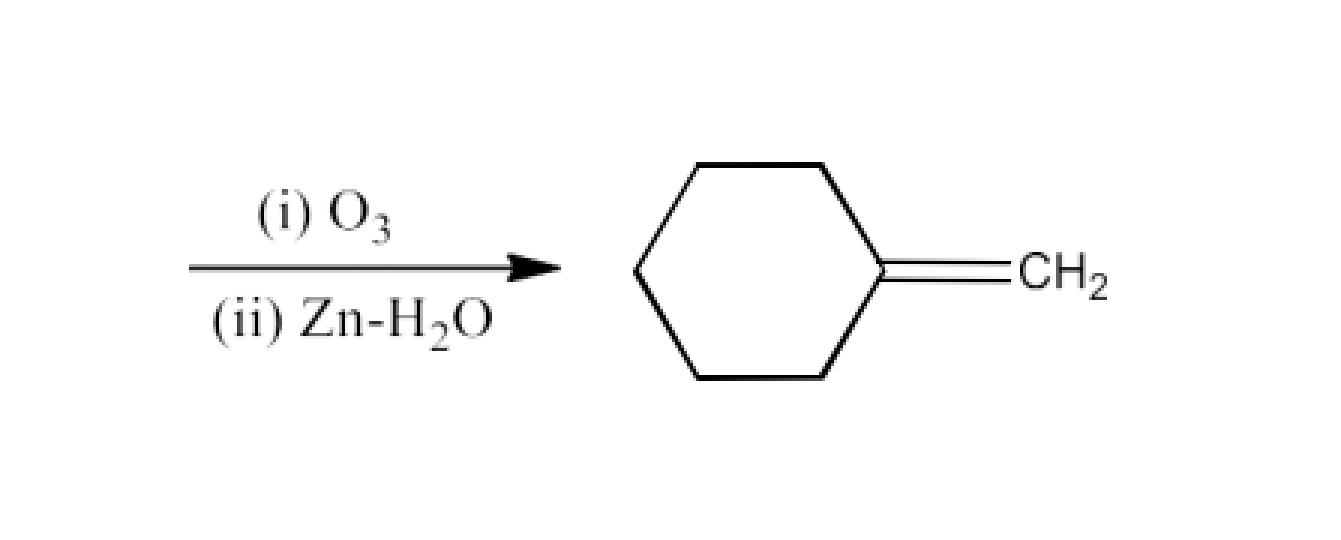
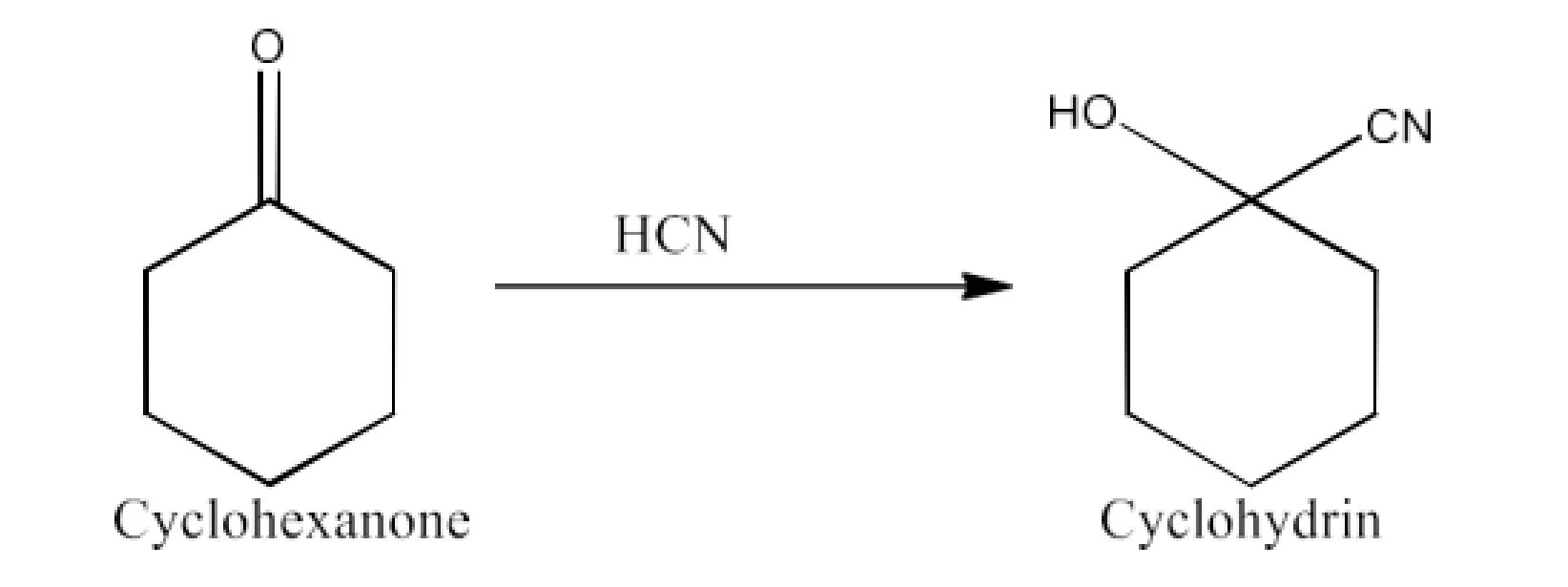
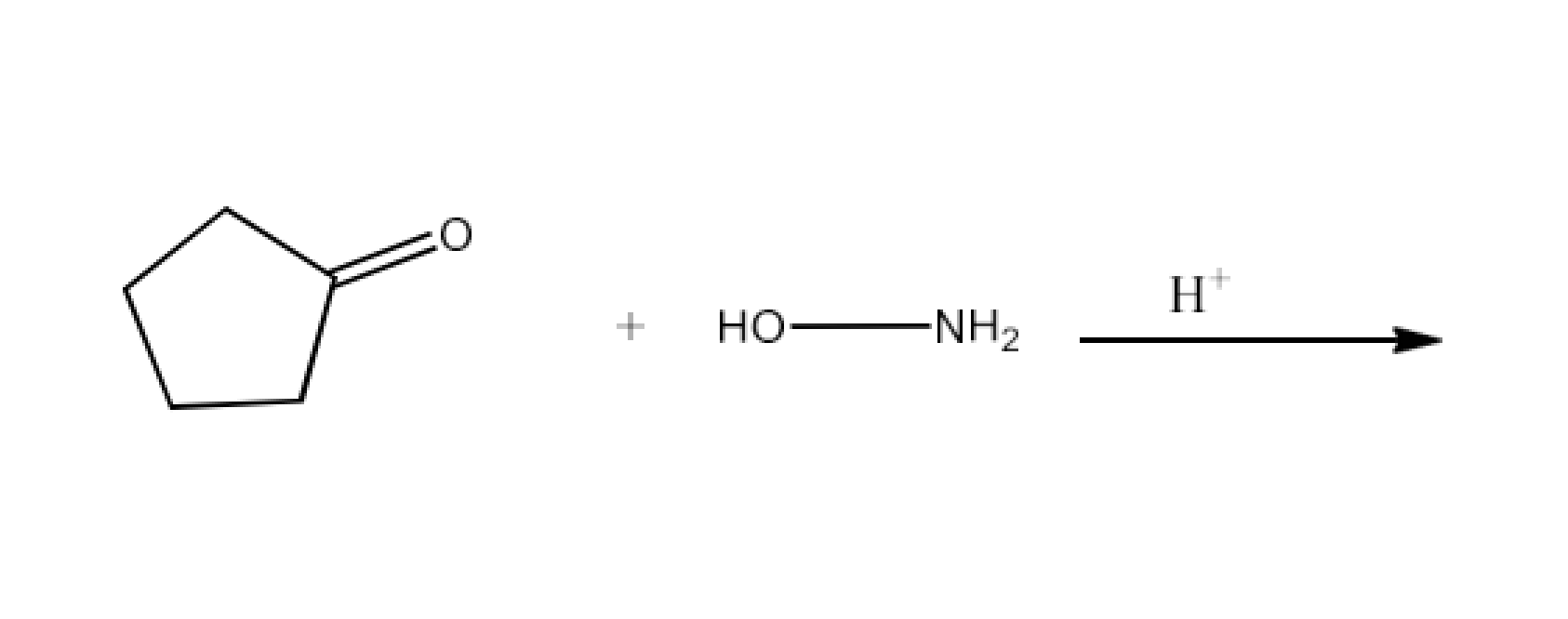
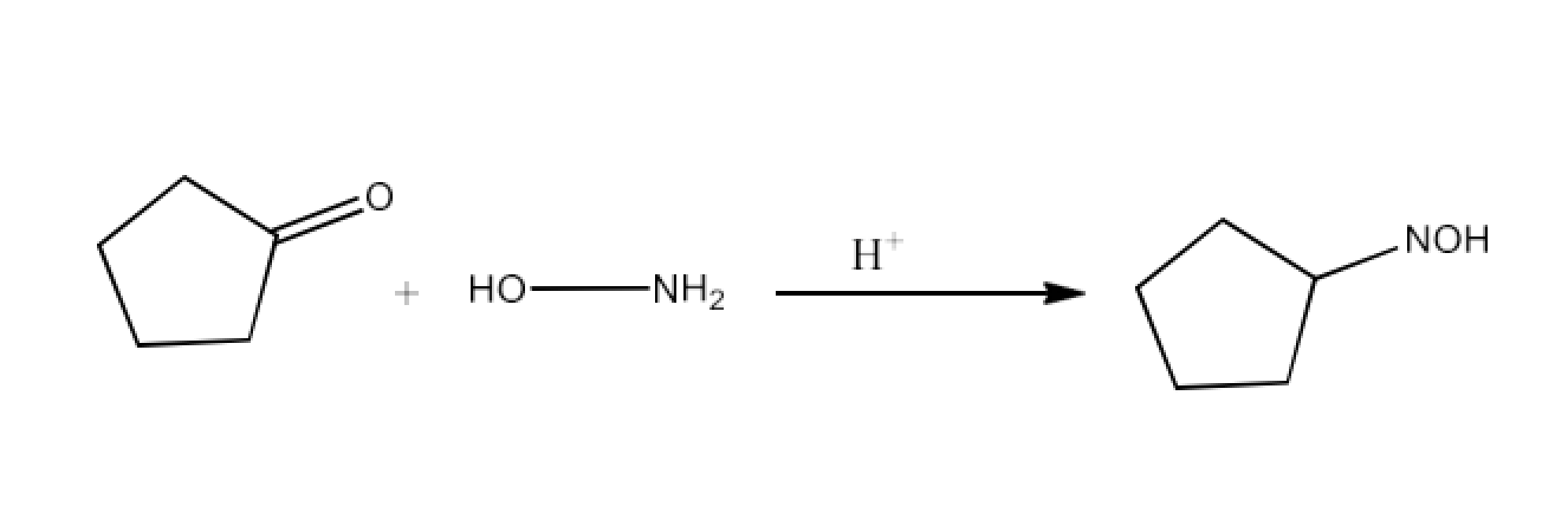
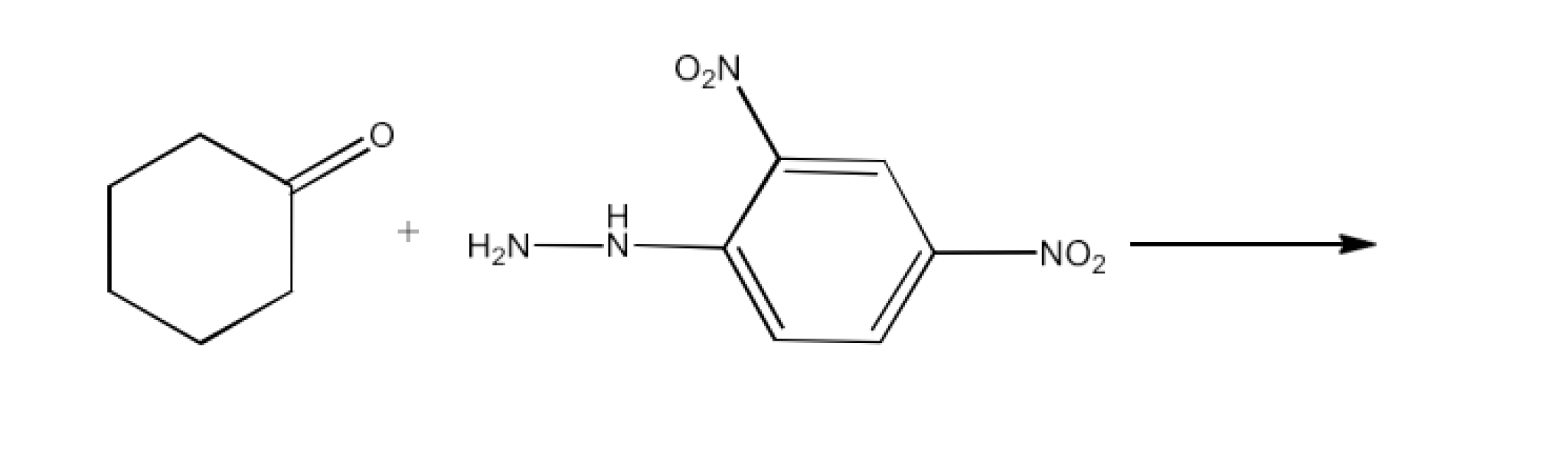
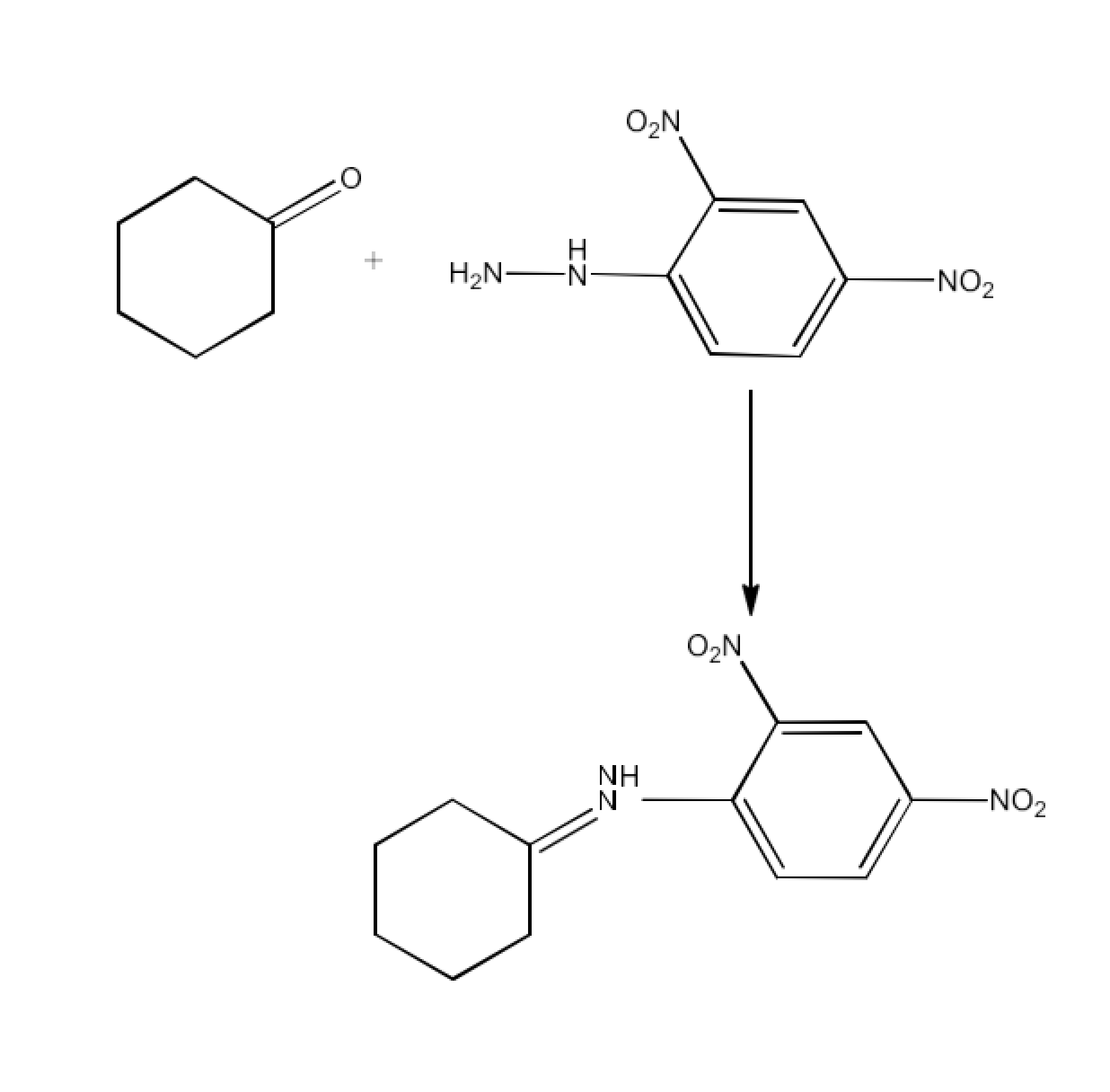
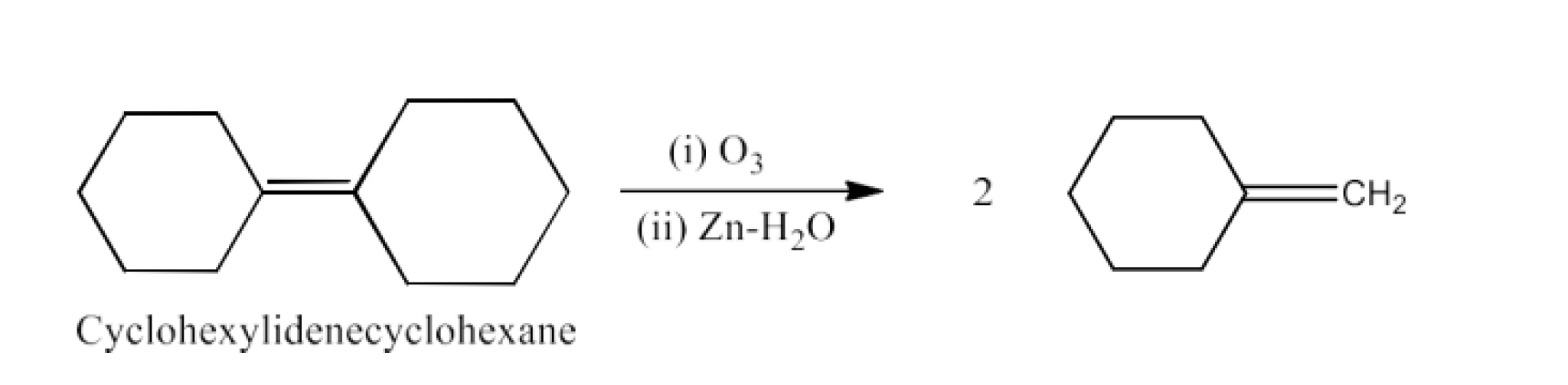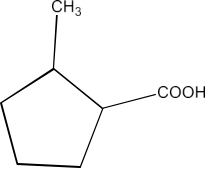Class 12 Chapter 8 Aldehydes Ketones and Carboxylic Acids NCERT Solutions FREE PDF Download





























FAQs on NCERT Solutions for Class 12 Chemistry Chapter 8 Aldehydes Ketones and Carboxylic Acids
1. What Should I Prepare from Aldehydes, Ketones, and Carboxylic Acids?
Students, while preparing chapter twelve for their boards, should at first go through the previous year’s papers. This will help them to find out the most relevant questions for this exam. Once they are done with this, they should start their preparation.
They should emphasise on the properties of Aldehyde, Ketones, and Carboxylic Acids. They should also practice their reactions regularly. While doing so, they should cross-check it from NCERT Solutions for Class 12 Chemistry aldehydes and ketones. Students should practice the exercises more than once. This will help them to identify their mistakes and rectify them well before exams.
2. Why Should I Refer to Chemistry Aldehydes ketones and Carboxylic acids NCERT solutions?
To get good grades in their boards or any other competitive exam, students should refer to more than one source for their preparation. Moreover, they should also take help of a solutions book. These will help them to get comprehensive knowledge about the topics. They can also refer to these books to see the answer writing pattern for their boards.
Even students who want to prepare themselves should cross-check their answers with that of the solutions book. This will help them to identify their shortcomings, and thereby will be able to rectify it. However, they should ensure that these sources are reliable.
3. What are the Important Physical Properties of Aldehydes and Ketones?
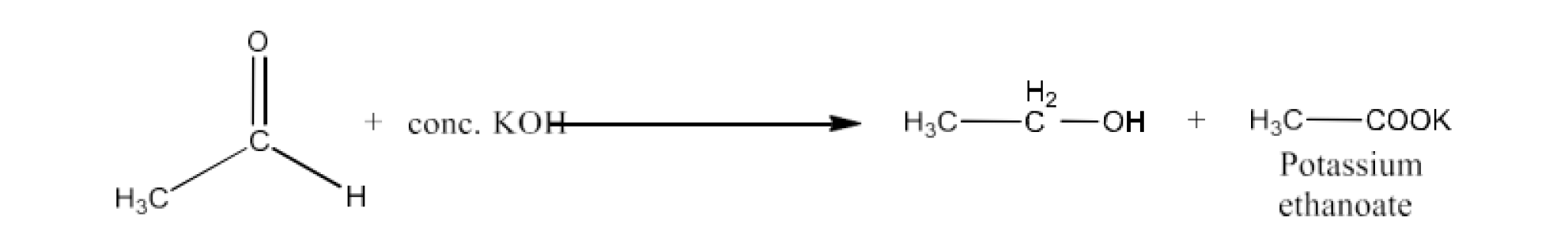
Aldehydes and Ketones are compounds that contain carbonyl groups. The boiling point of these organic compounds depends on the intermolecular force. It increases with the increase in carbon atoms. Moreover, both these compounds are polar and also soluble in water.
While preparing for exams, students should emphasise on the properties of these compounds. Besides these physical properties, they should also study the chemical compounds for scoring better grades. To get a comprehensive idea about the properties of these compounds, they can refer to Aldehydes ketones and Carboxylic acids NCERT solutions. This will help them to get a better understanding of these compounds and carboxylic compounds also.
4. Where can I download NCERT solutions class 12 chemistry Aldehydes ketones and carboxylic acids?
You can download NCERT Solutions For Class 12 Chemistry Chapter 12 from Vedantu’s website free of cost-
Visit the page Chemistry Class 12and select the chapter Adehydes, Ketones and Carboxylic Acids.
The page will show the exercise questions. Click on the questions for which you want to see the solutions.
Click on the link for the exercise PDF.
NCERT solutions class 12 chemistry aldehydes ketones and carboxylic acids PDF will be displayed on your screen. Click on the ‘Download PDF ‘ option to download and save for offline use.
5. What are Aldehydes, Ketones, and Carboxylic acids?
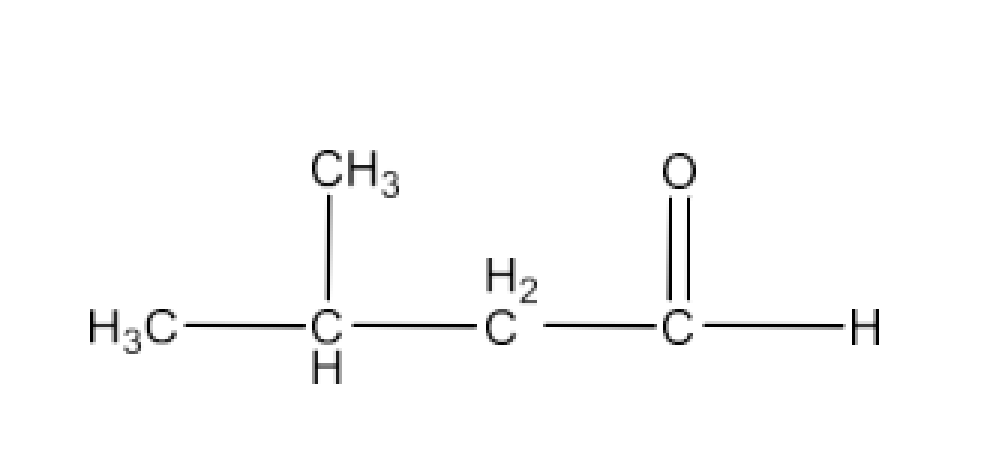
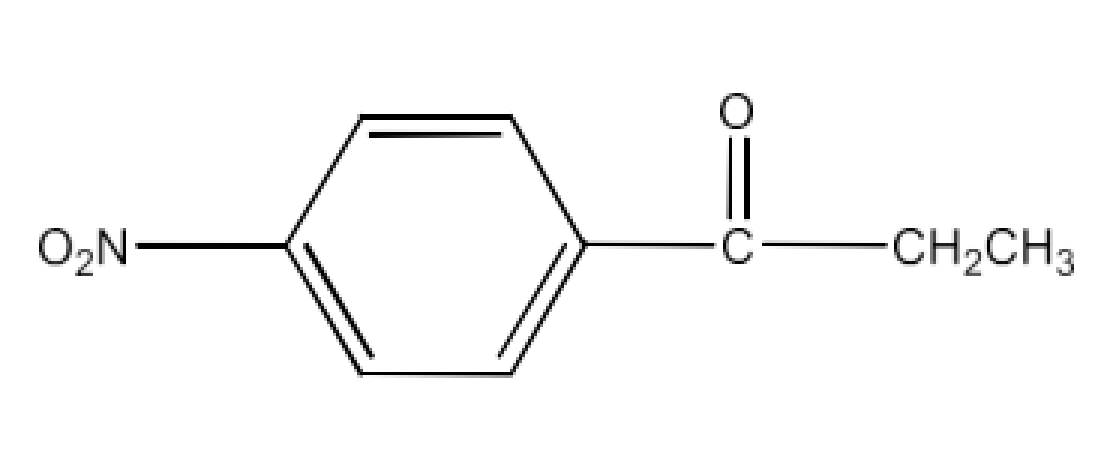
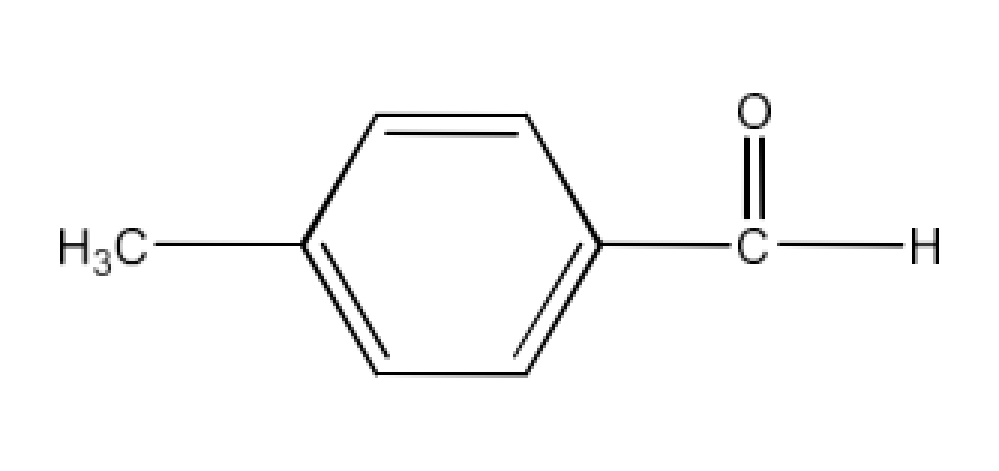
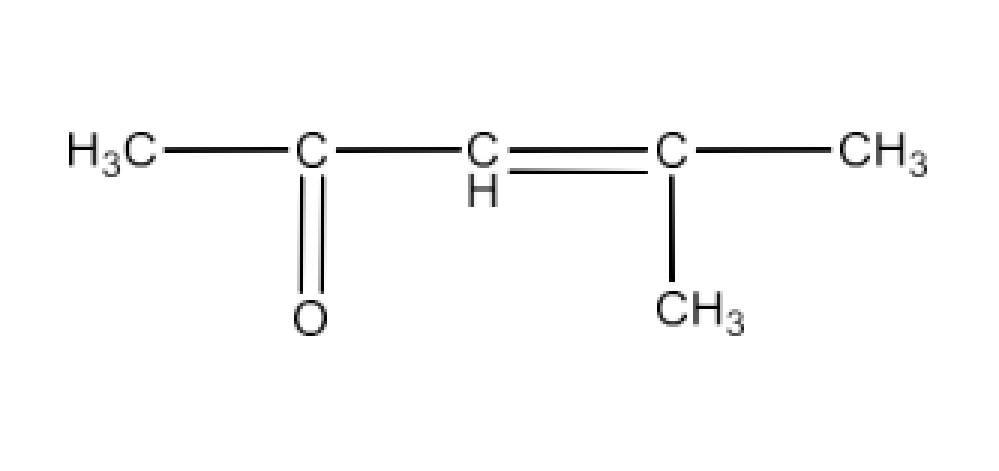
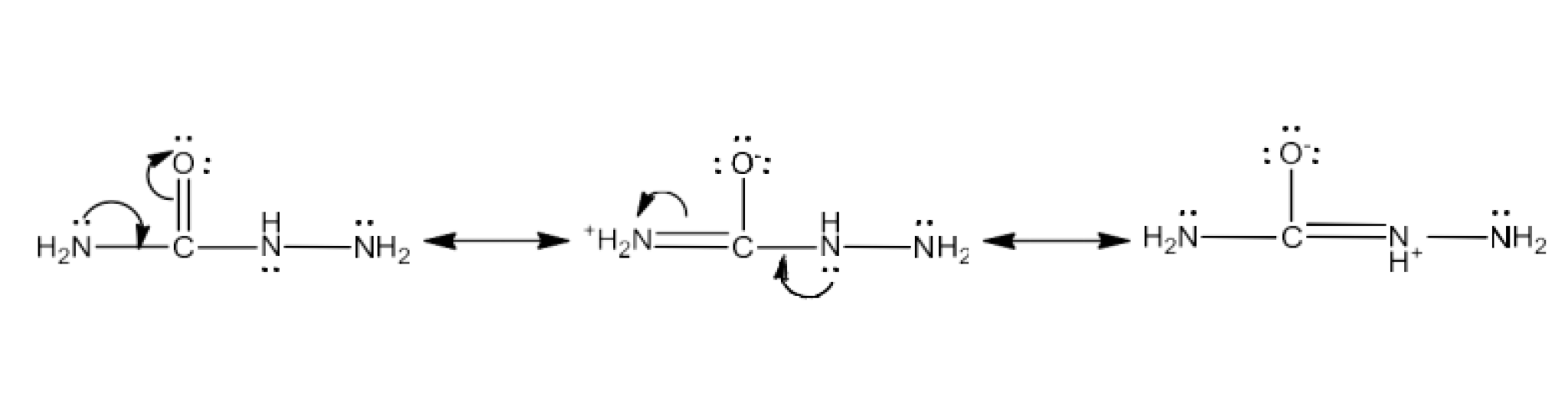
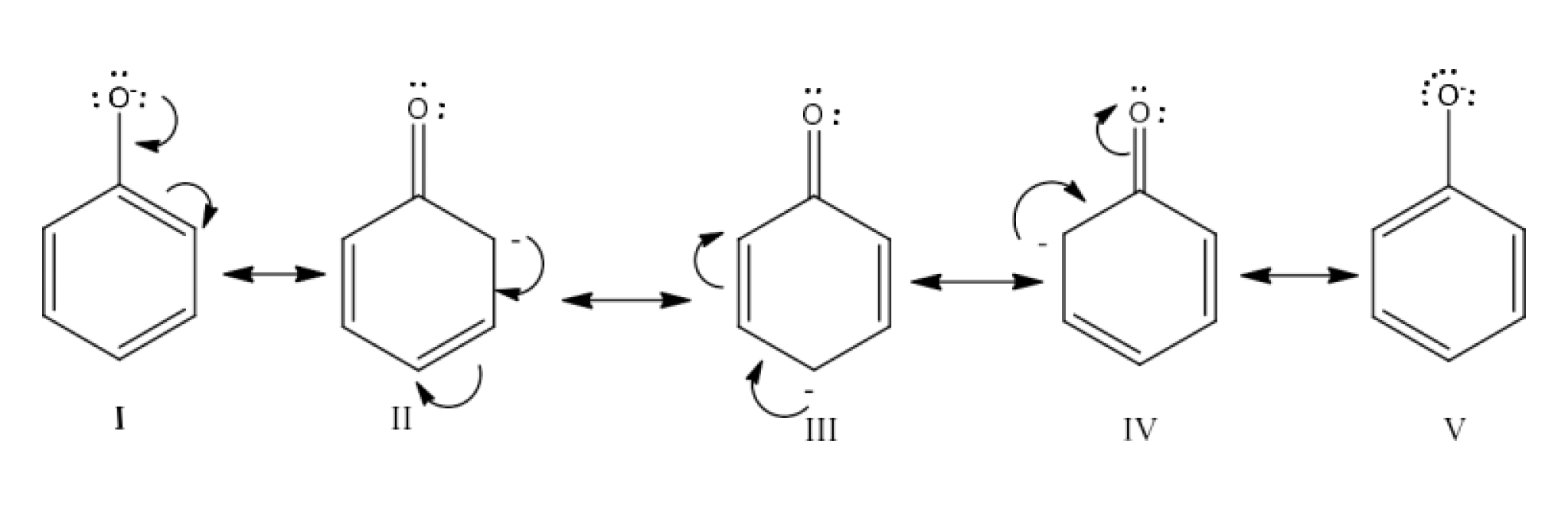
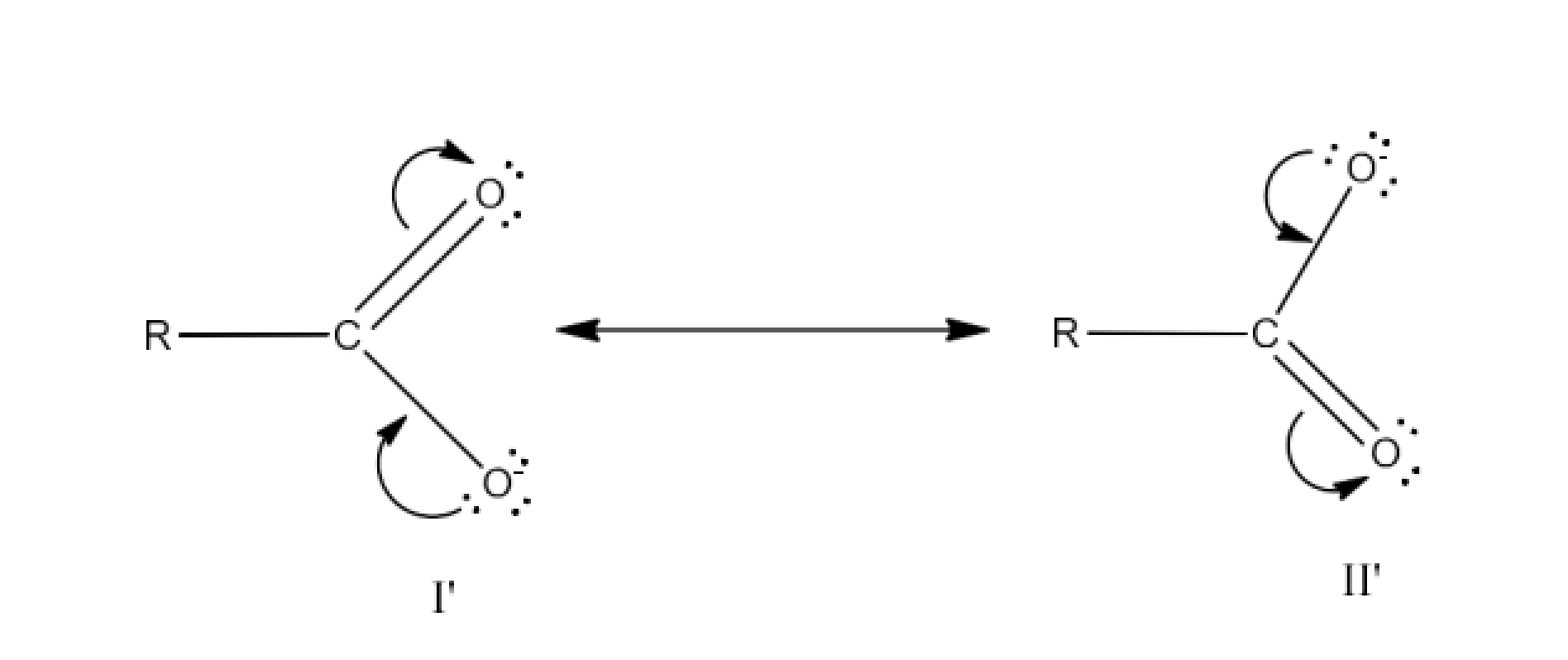
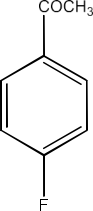
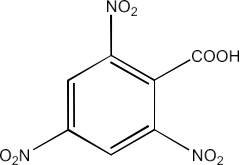
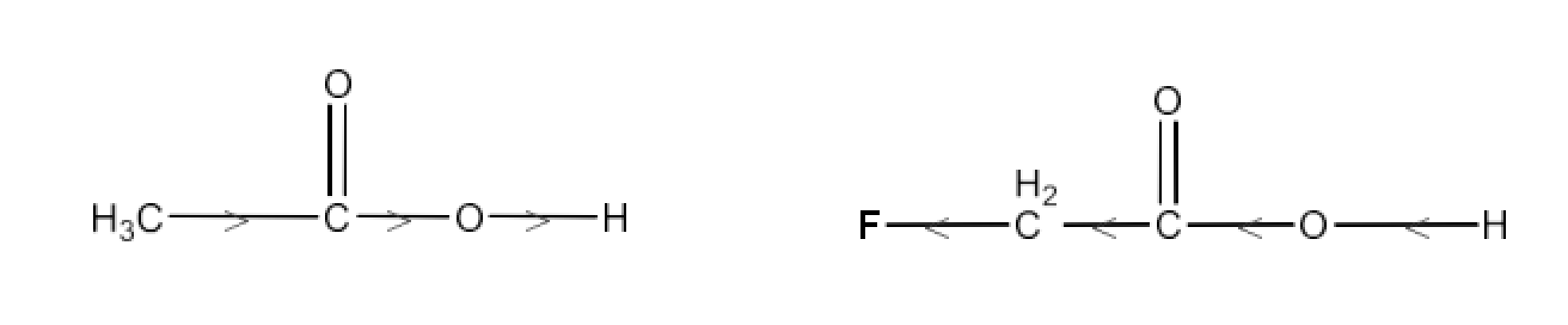
Carbonyl compounds contain a carbon-oxygen double bond called the carbonyl group. Aldehydes, ketones, and carboxylic acids belong to the class of carbonyl compounds. In aldehydes, the carbonyl group is bonded to carbon and hydrogen while in ketones, it is bonded to two carbon atoms. In all the carboxylic acids, the carbonyl group is attached to carbon or hydrogen and oxygen of hydroxyl moiety (-OH). They are all highly polar compounds. Refer to Vedantu’s aldehyde and ketone NCERT solutions to understand the concepts of this chapter at free of cost on the Vedantu app and the Vedantu website.
6. How to study organic chemistry for class 12 Chemistry?
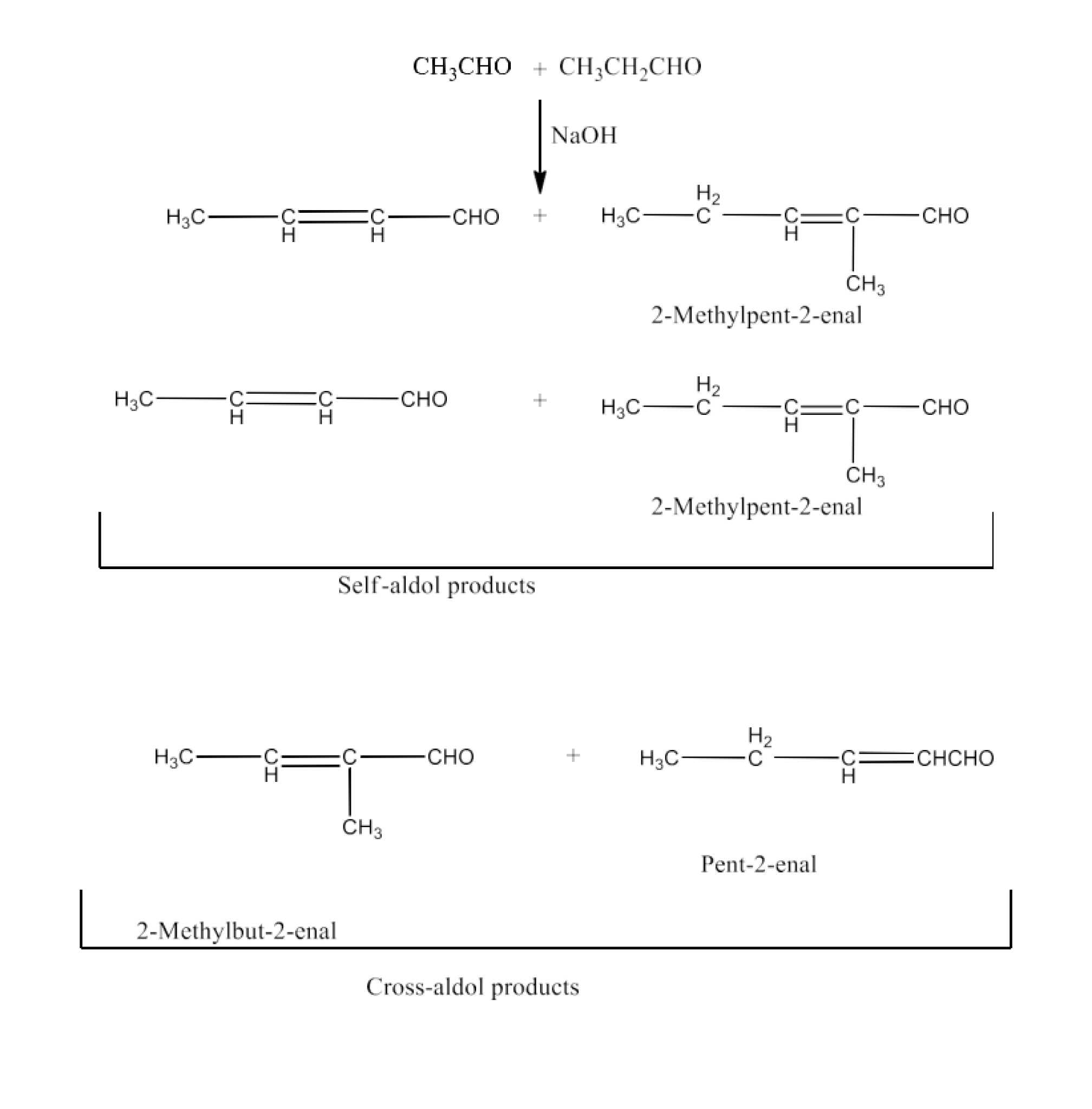
Organic chemistry forms a very important part of chemistry in Class 12. It forms the foundation and base for the subject for the upcoming years. It also gives a great basic understanding of all the competitive exams like JEE and NEET. Start by studying all the chapters in NCERT systematically and practicing the structures. Practice all the equations and reactions. Their physical and chemical properties are important and should be practiced well. Make sure you maintain a book of equations for the final revision.
7. What are the uses of aldehydes and ketones?
Aldehyde ketone and carboxylic acids NCERT solutions forms a very important part of chemistry in Class 12. It forms the foundation and base for the subject for the upcoming years. It also gives a great basic understanding of all the competitive exams like JEE and NEET. Start by studying all the chapters in NCERT systematically and practising the structures. Practise all the equations and reactions. Their physical and chemical properties are important and should be practised well. Make sure you maintain a book of equations for the final revision.
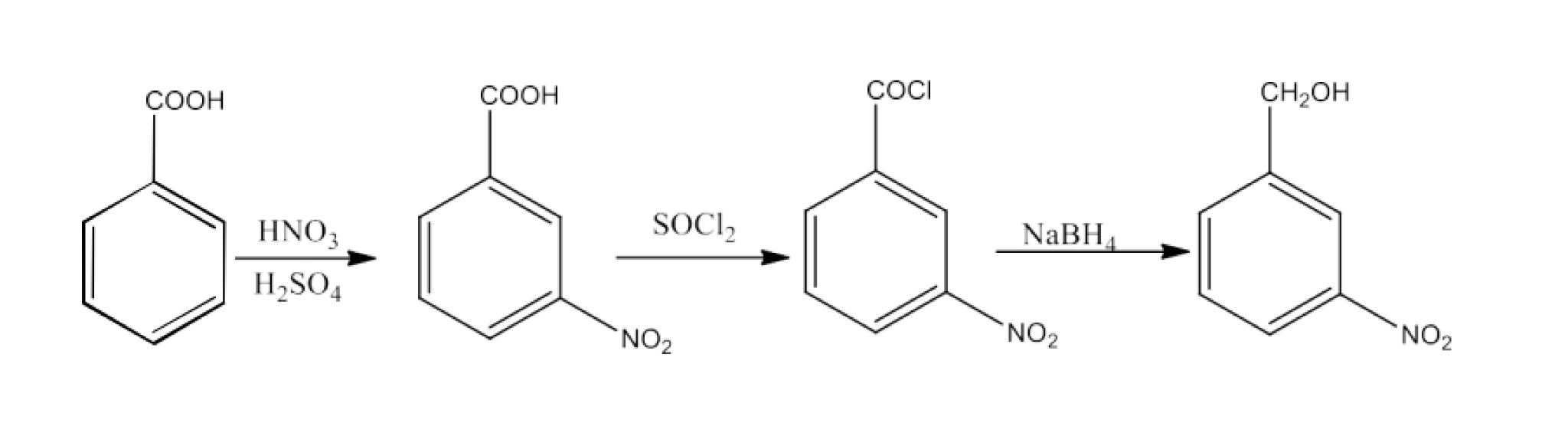
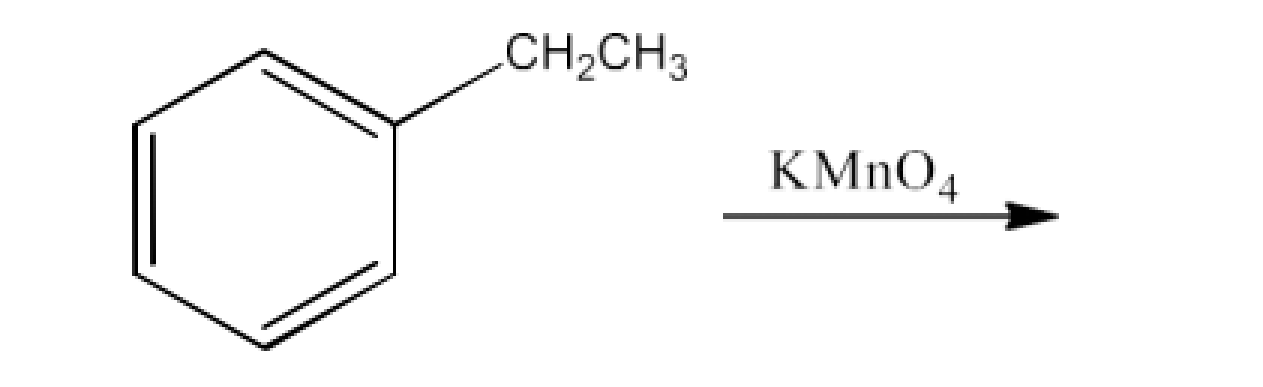
8. What are the methods for carboxylic acid preparation?
Carboxylic acids can be prepared by six methods-
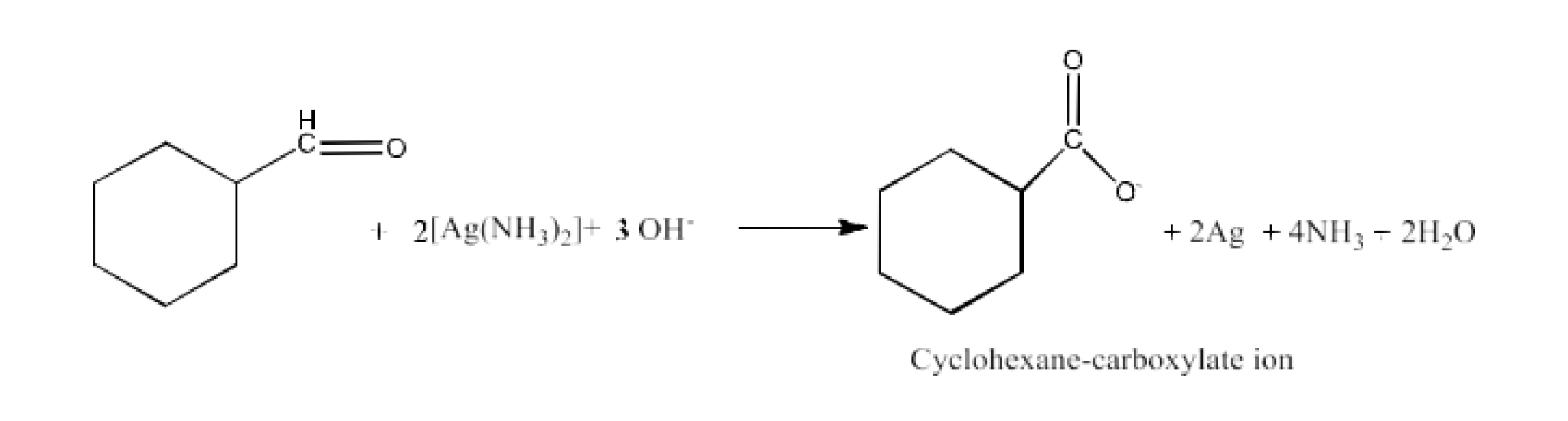
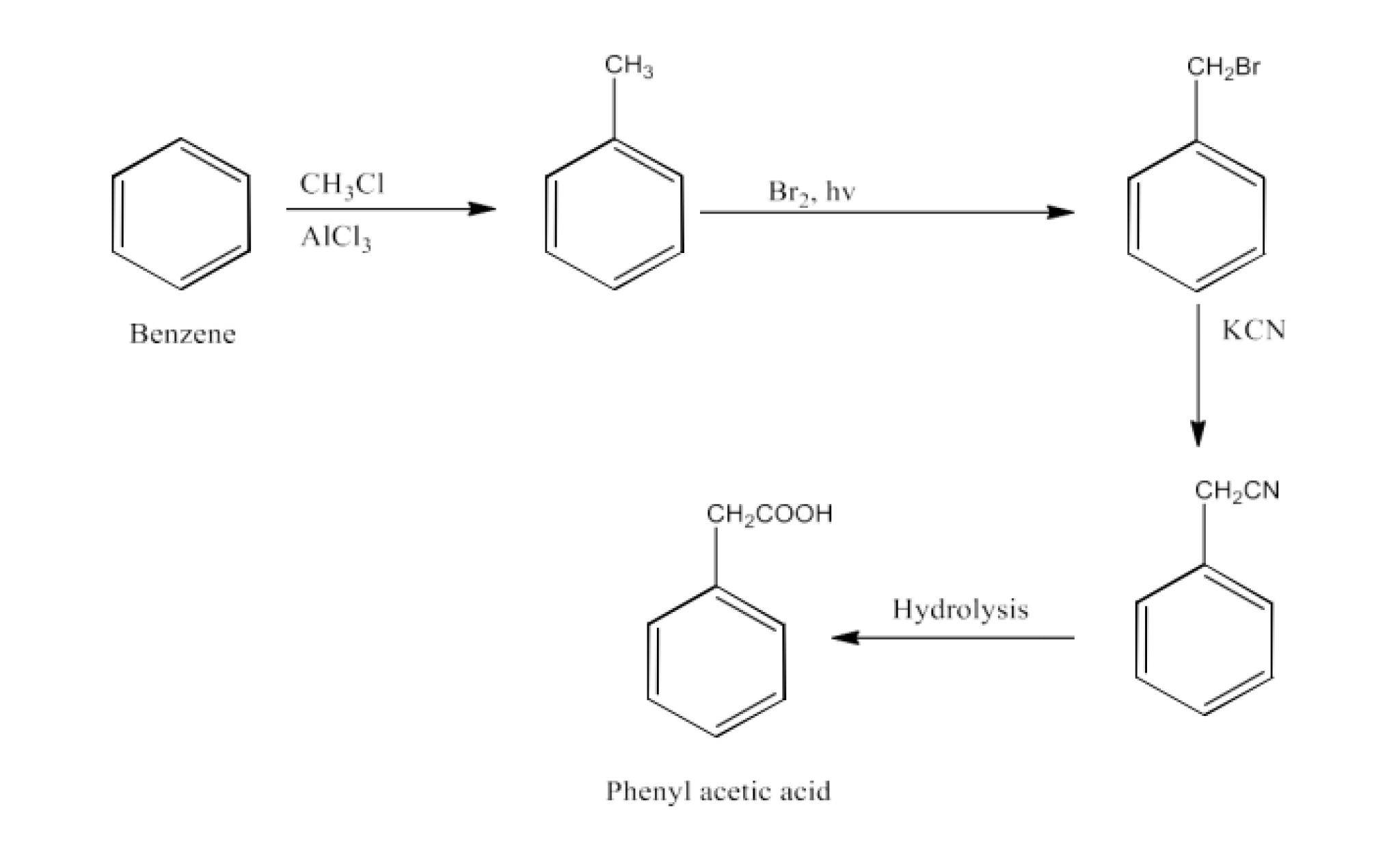
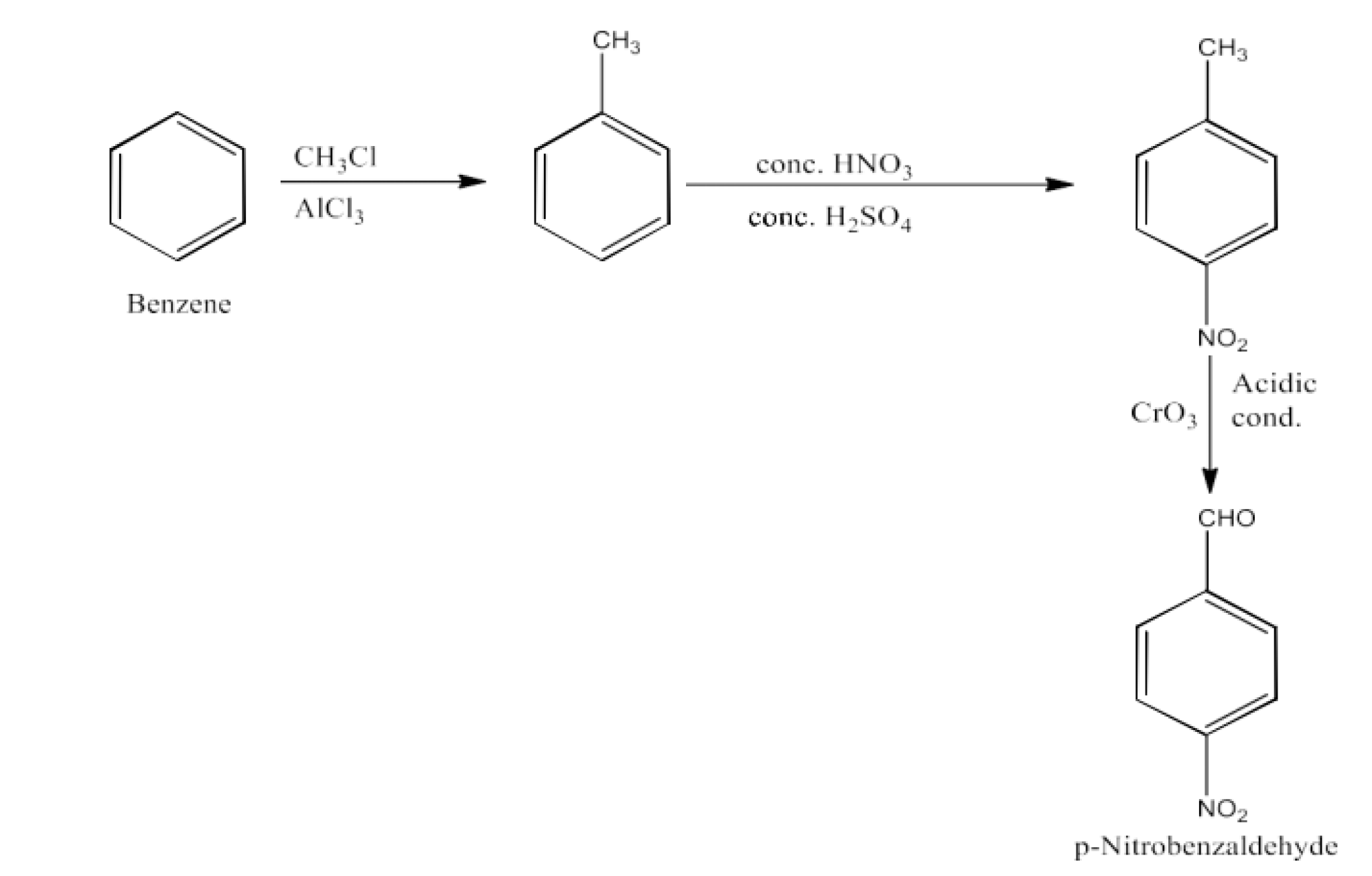
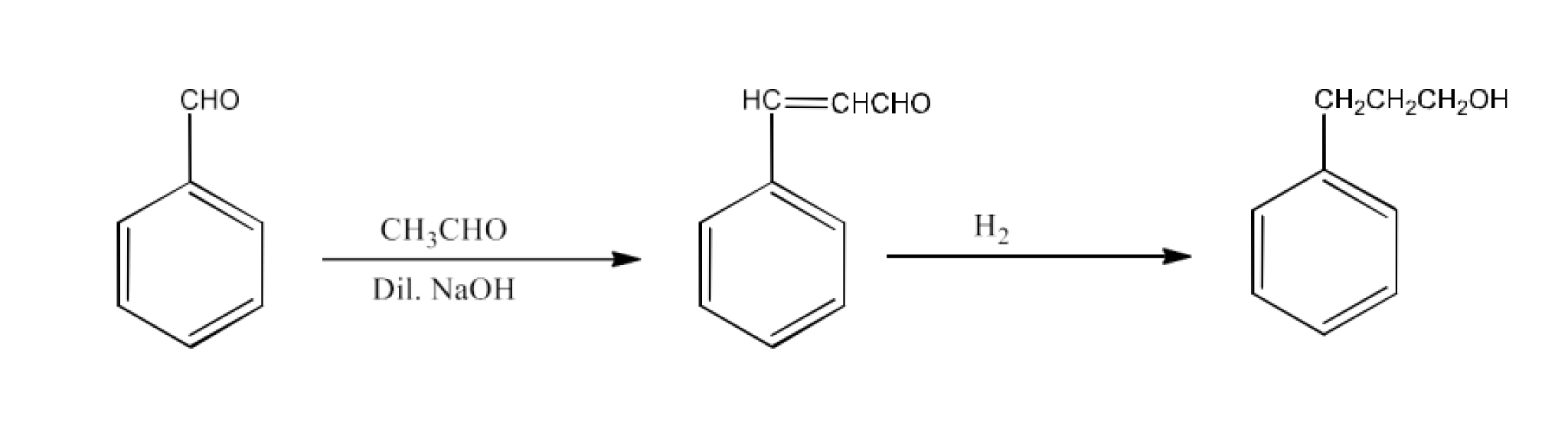
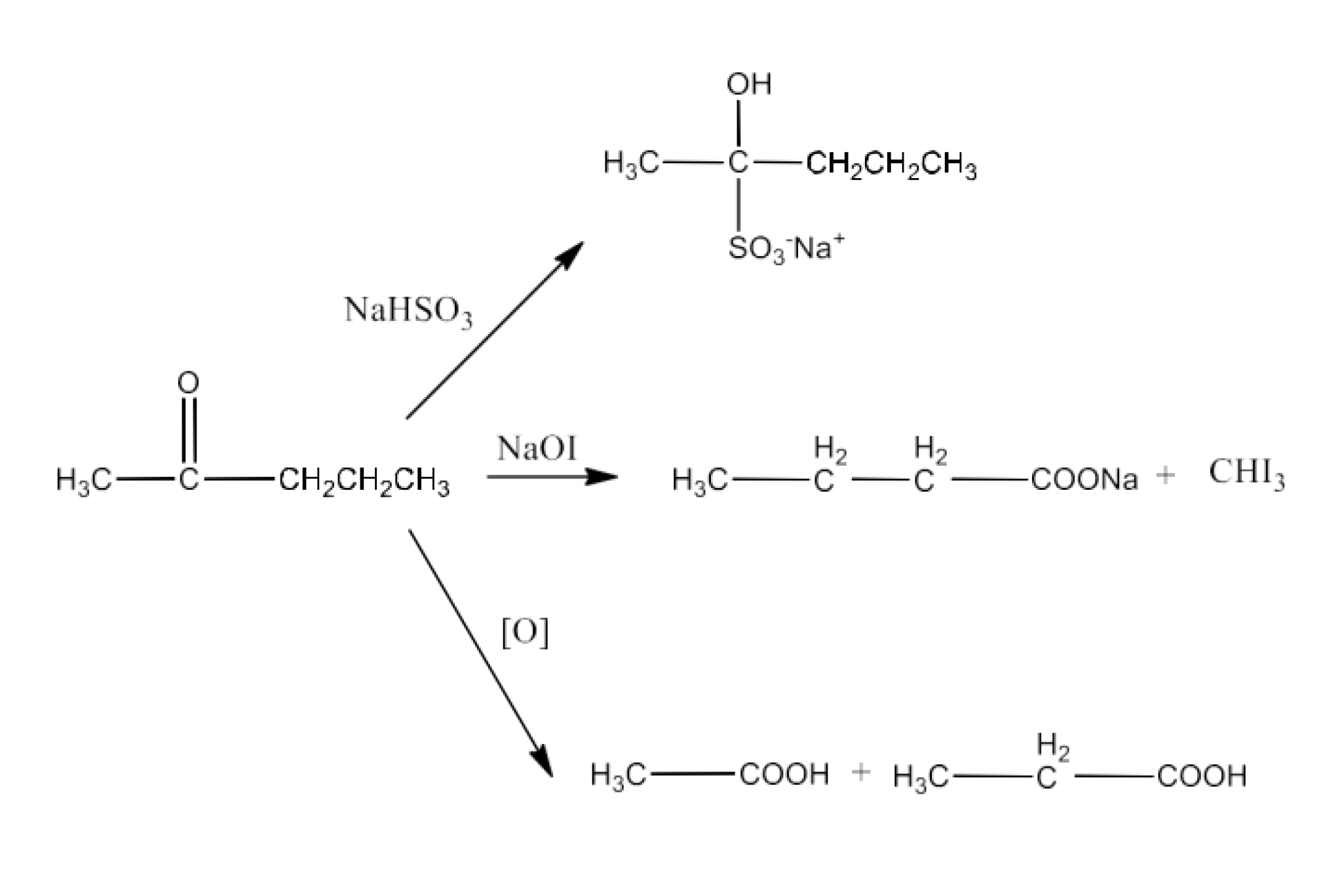
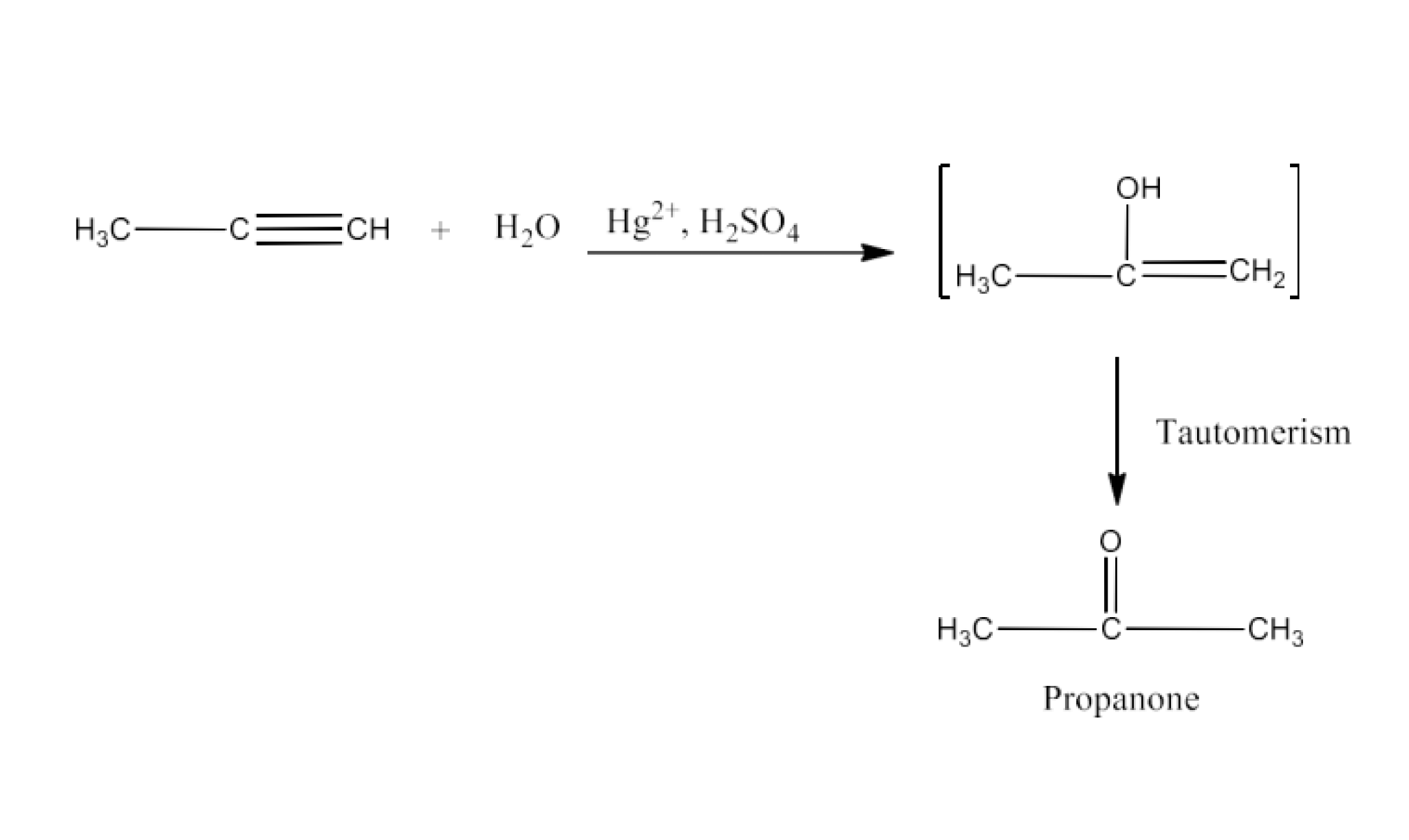
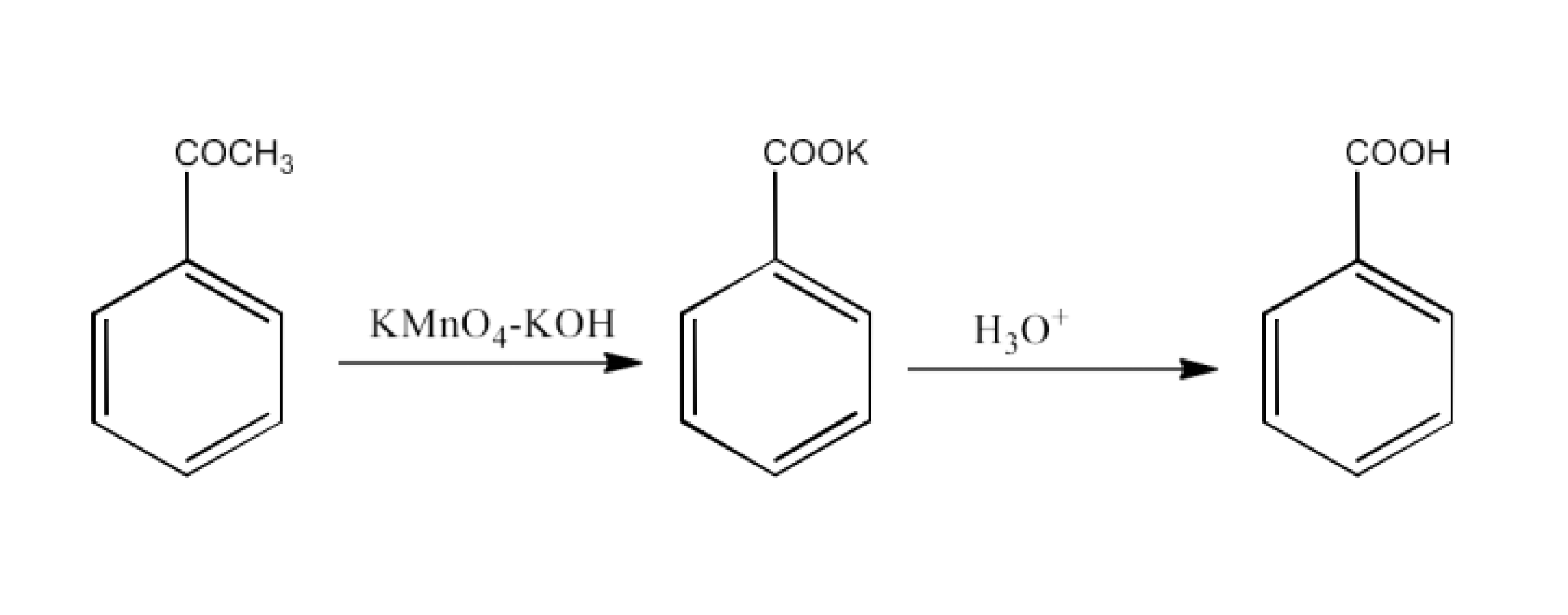
From primary alcohols and aldehydes- primary alcohols are oxidized to form carboxylic acids using oxidizing agents. They are also prepared from aldehydes using mild oxidizing agents.
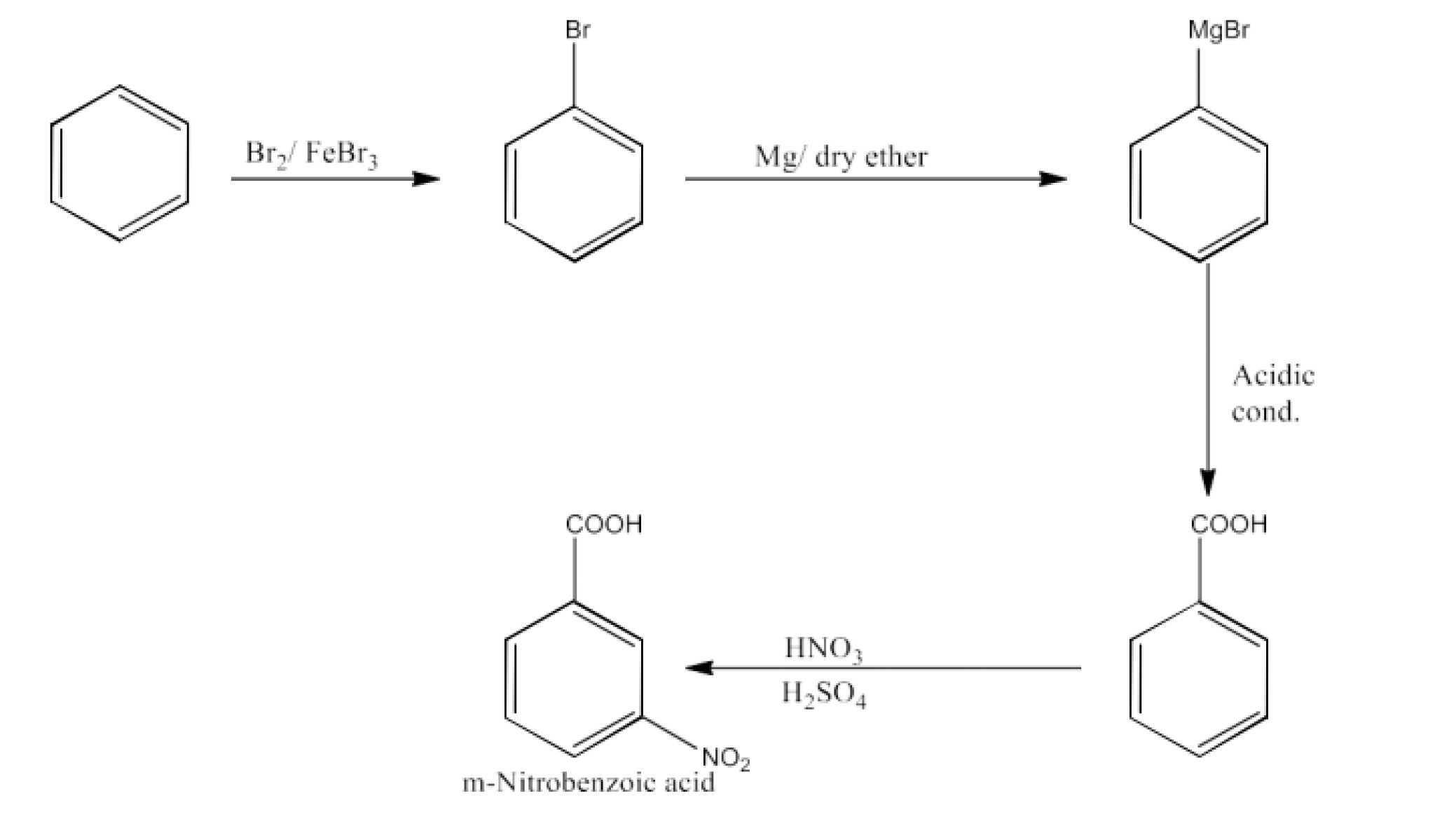
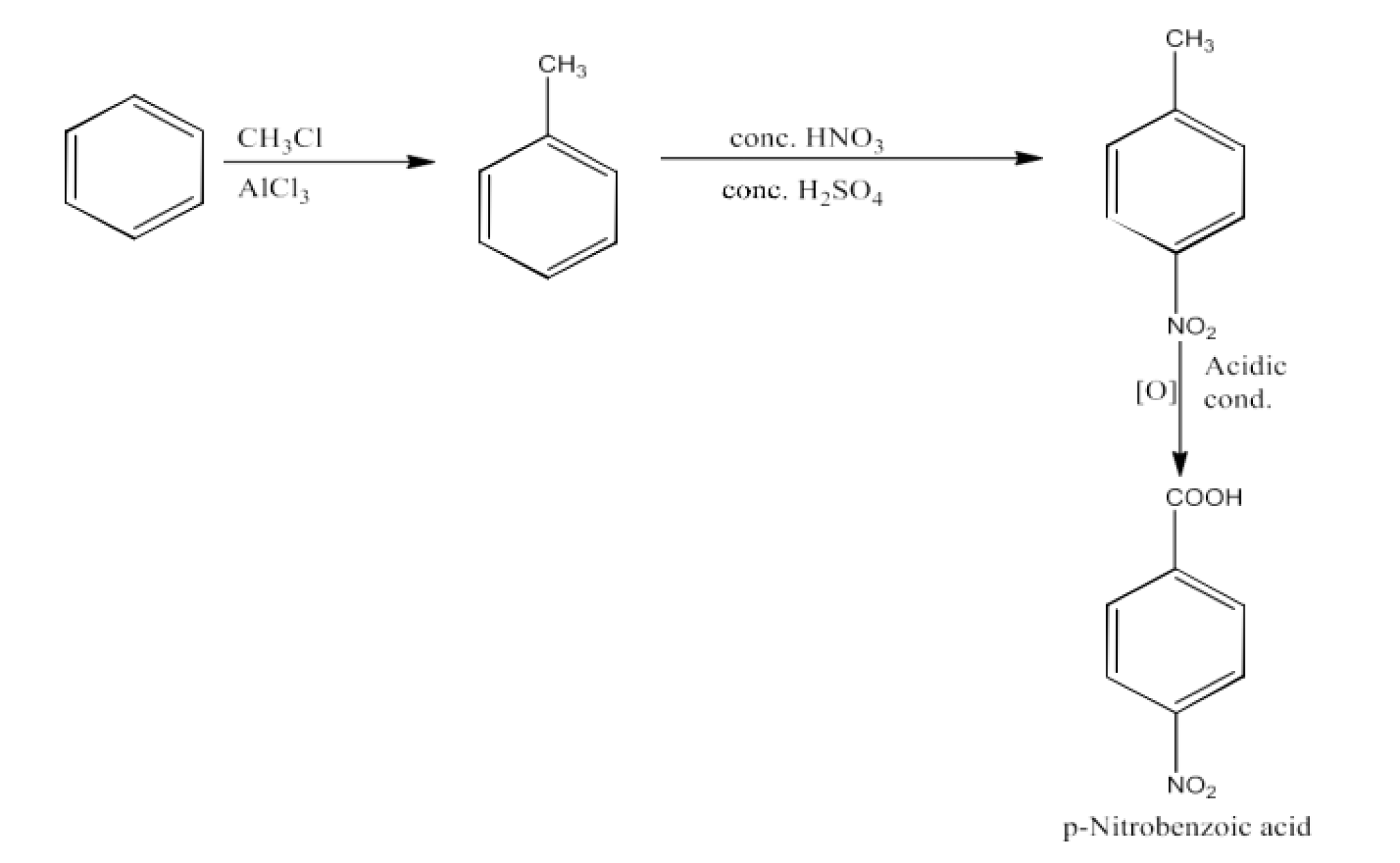
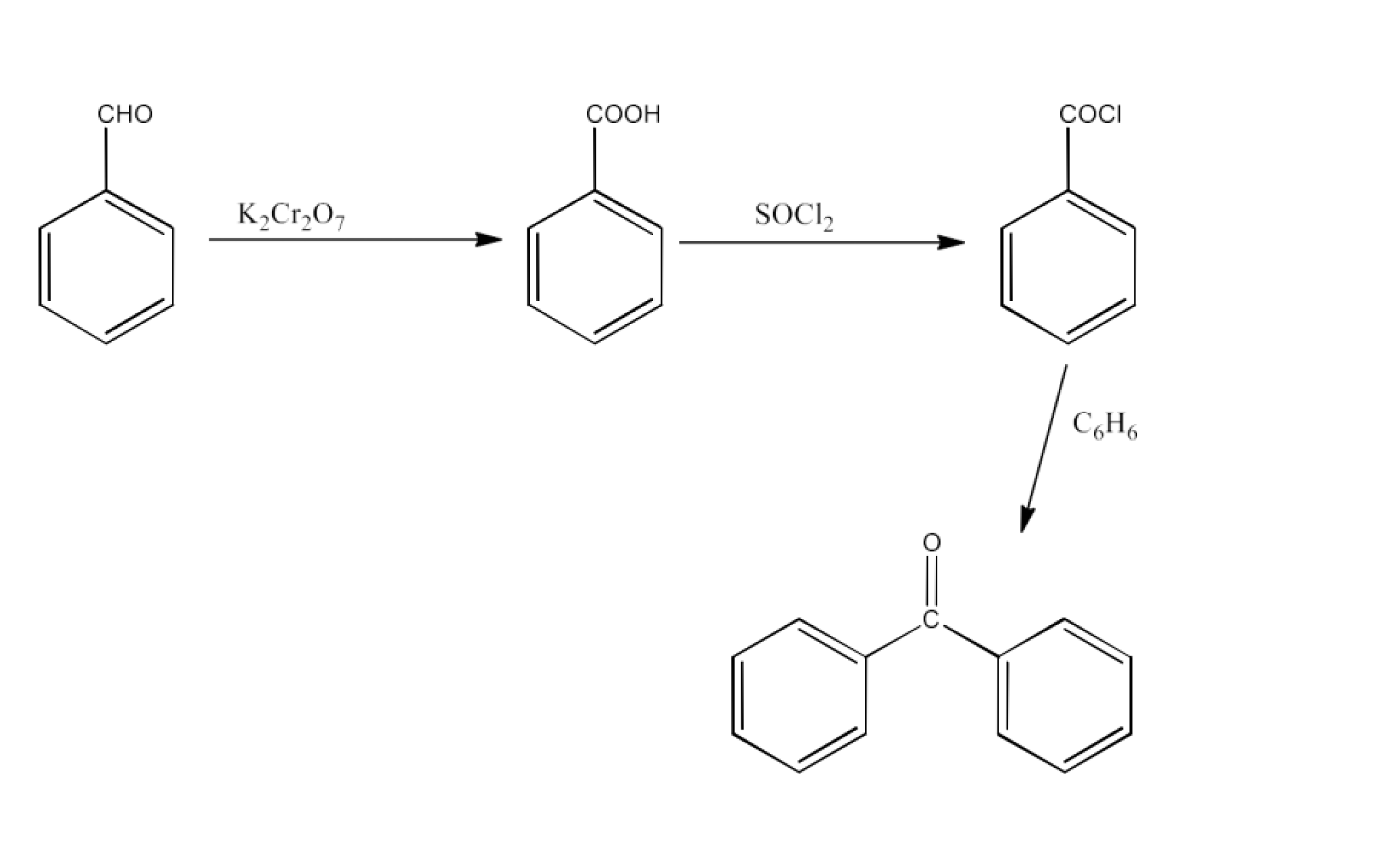
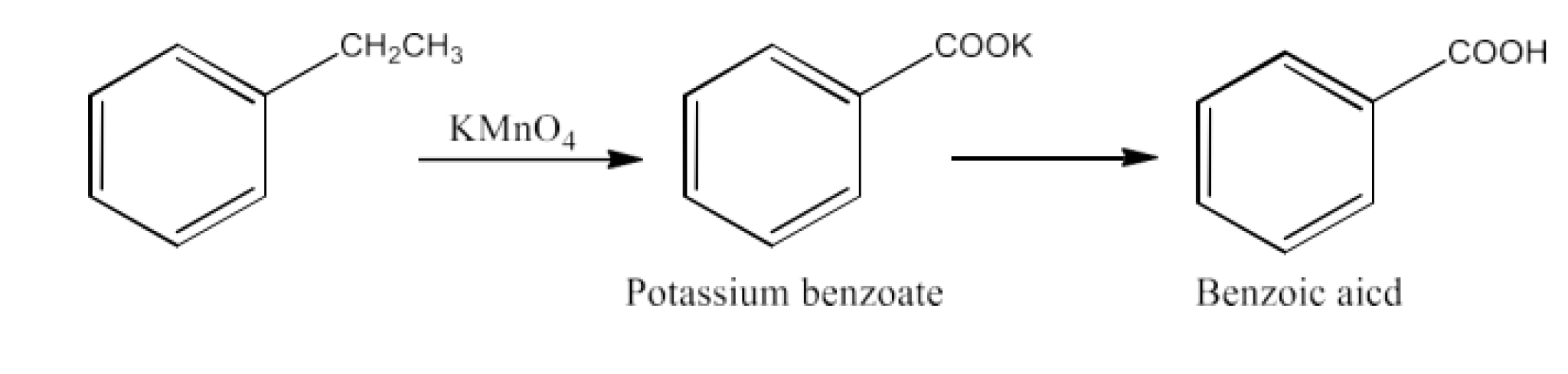
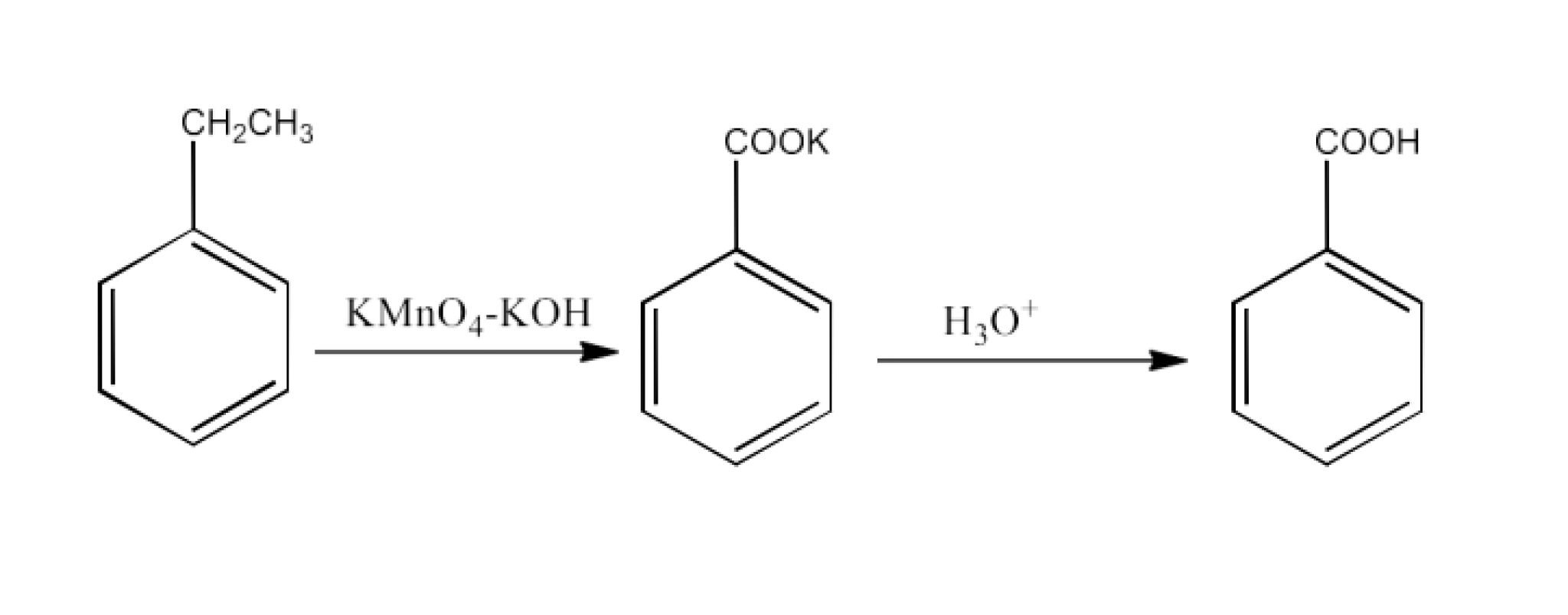
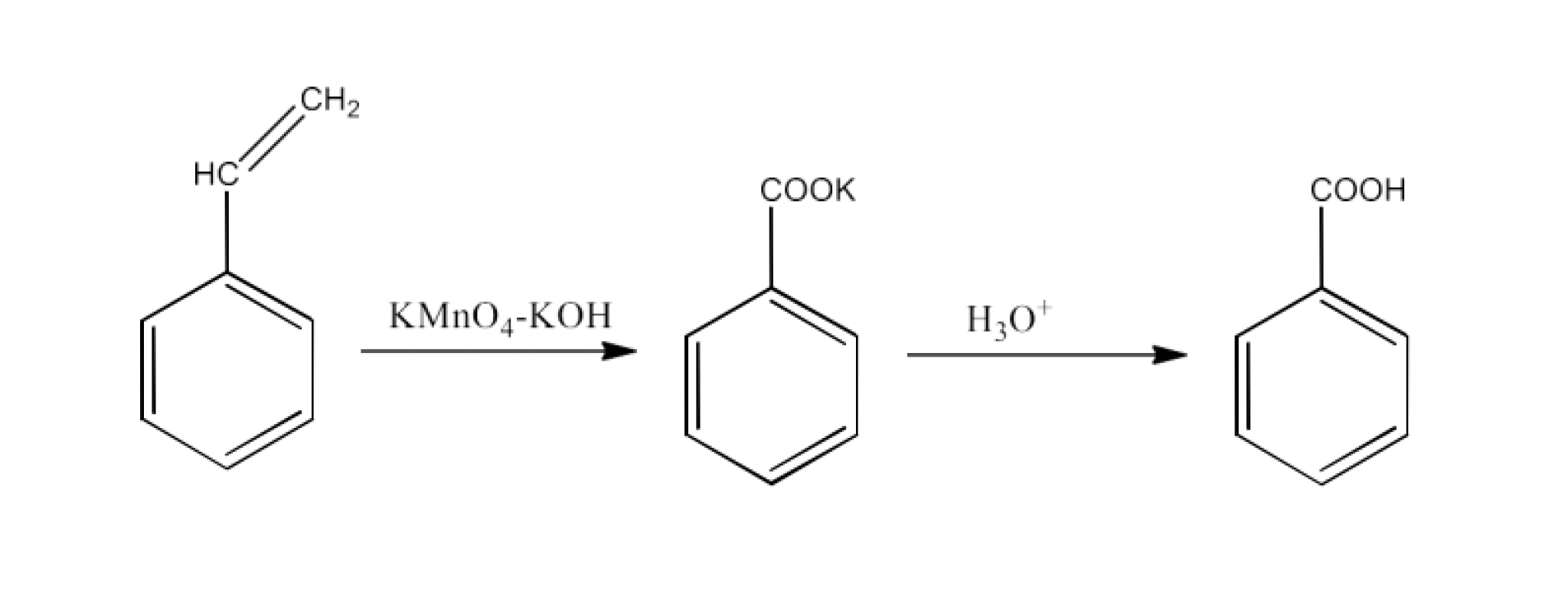
From alkylbenzenes- preparation of aromatic carboxylic acids
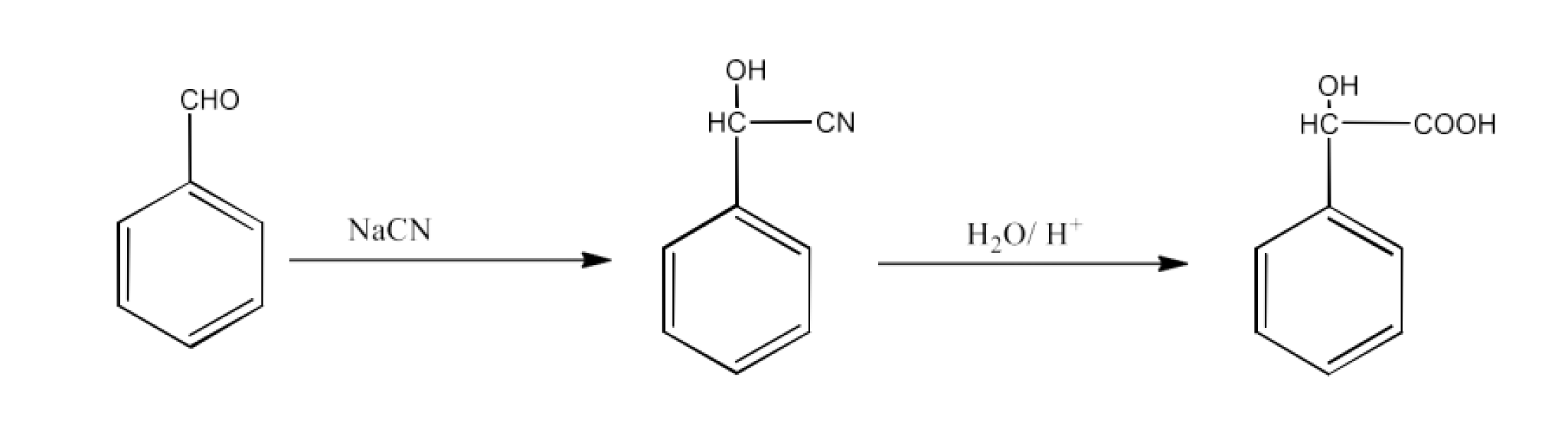
From nitriles and amides- Nitriles are first hydrolyzed to amides and then converted to acids.
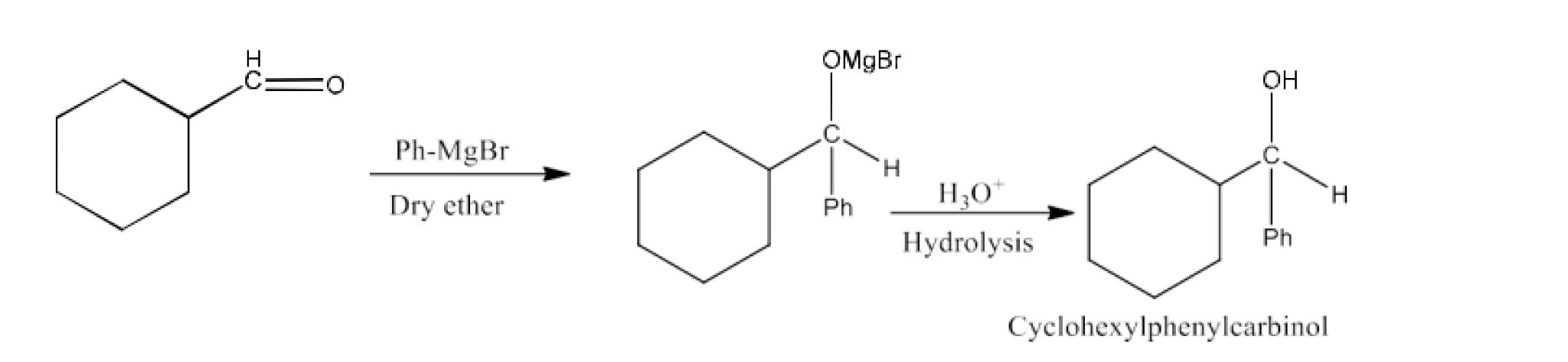
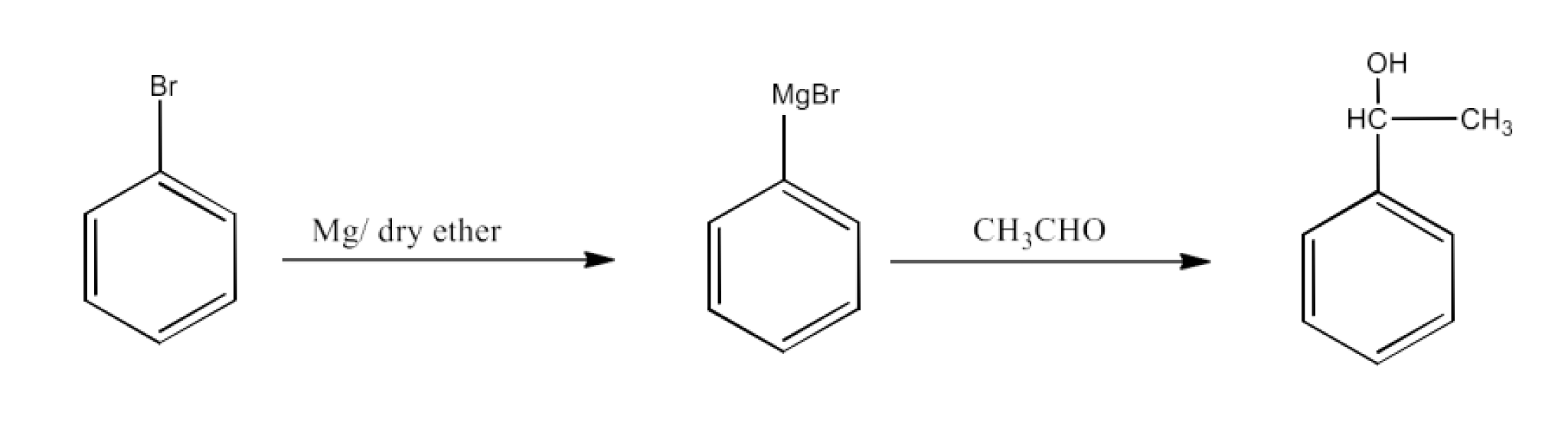
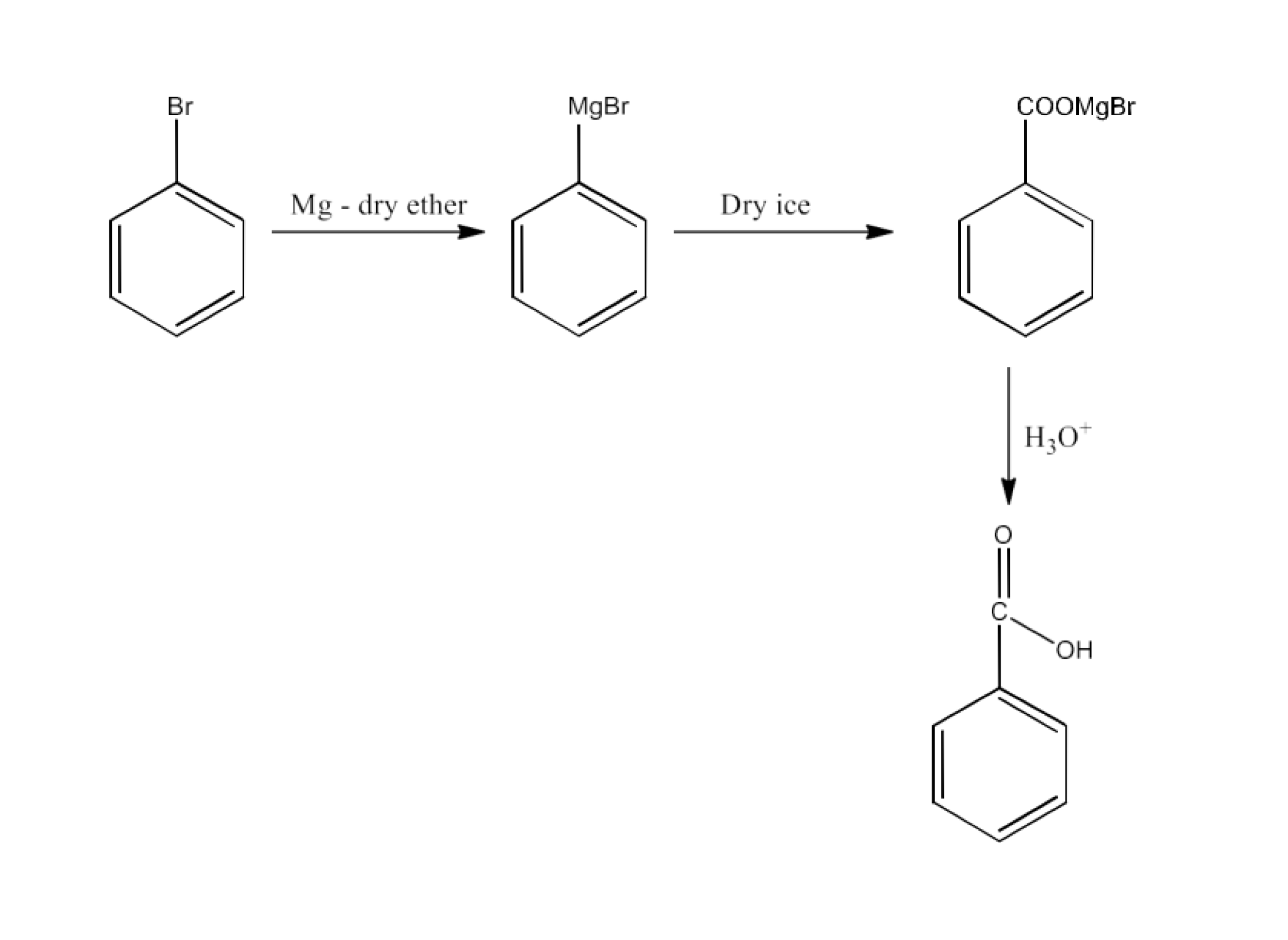
From Grignard reagents
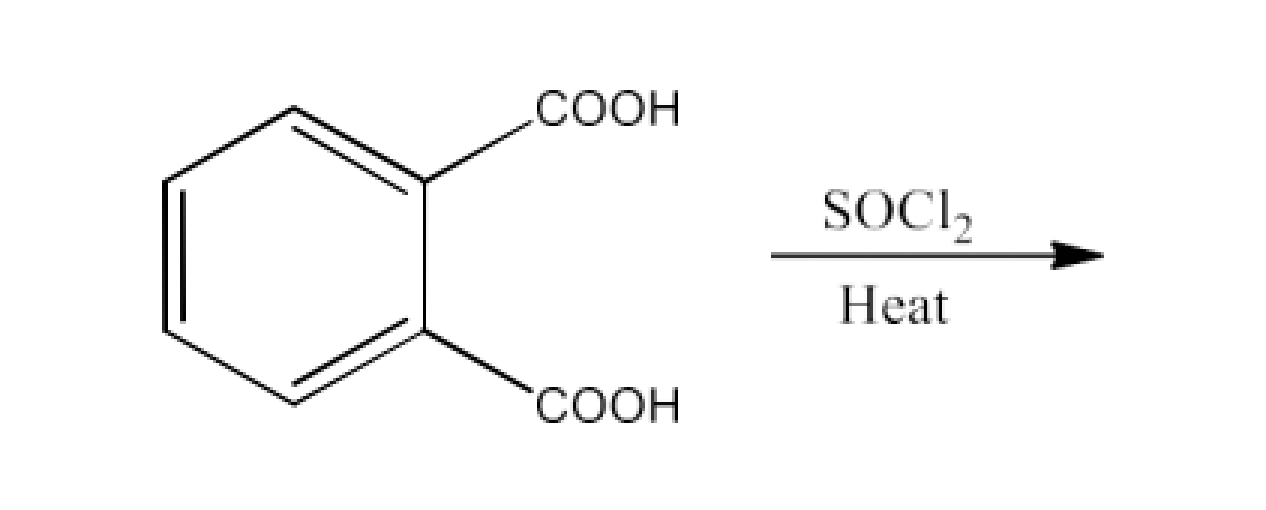
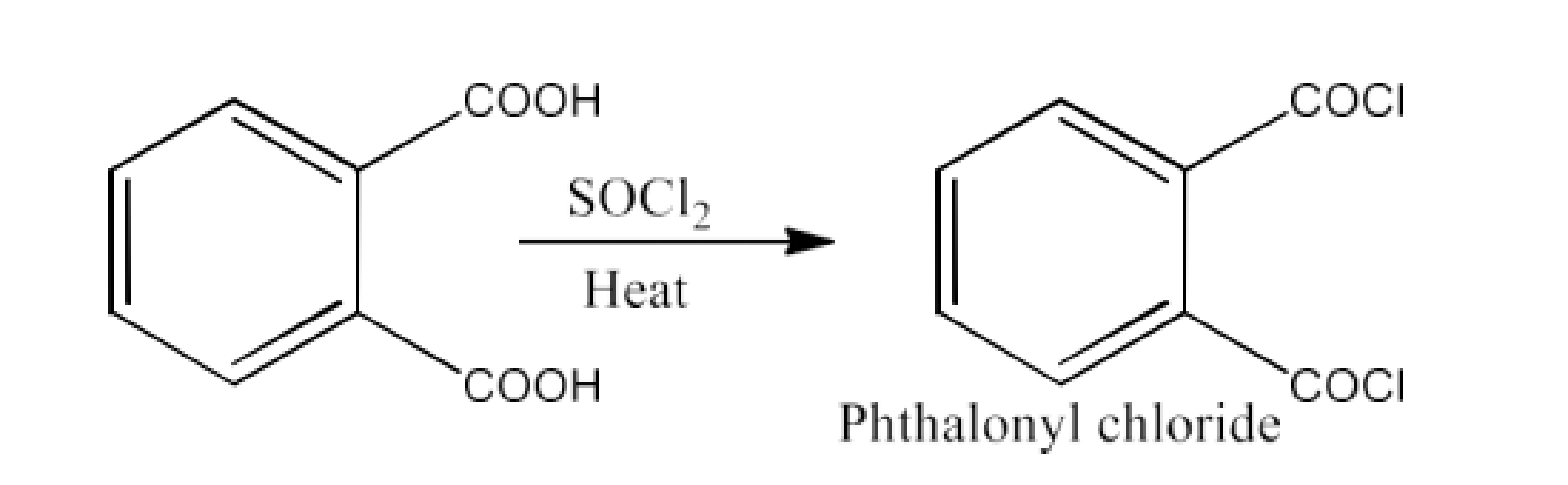
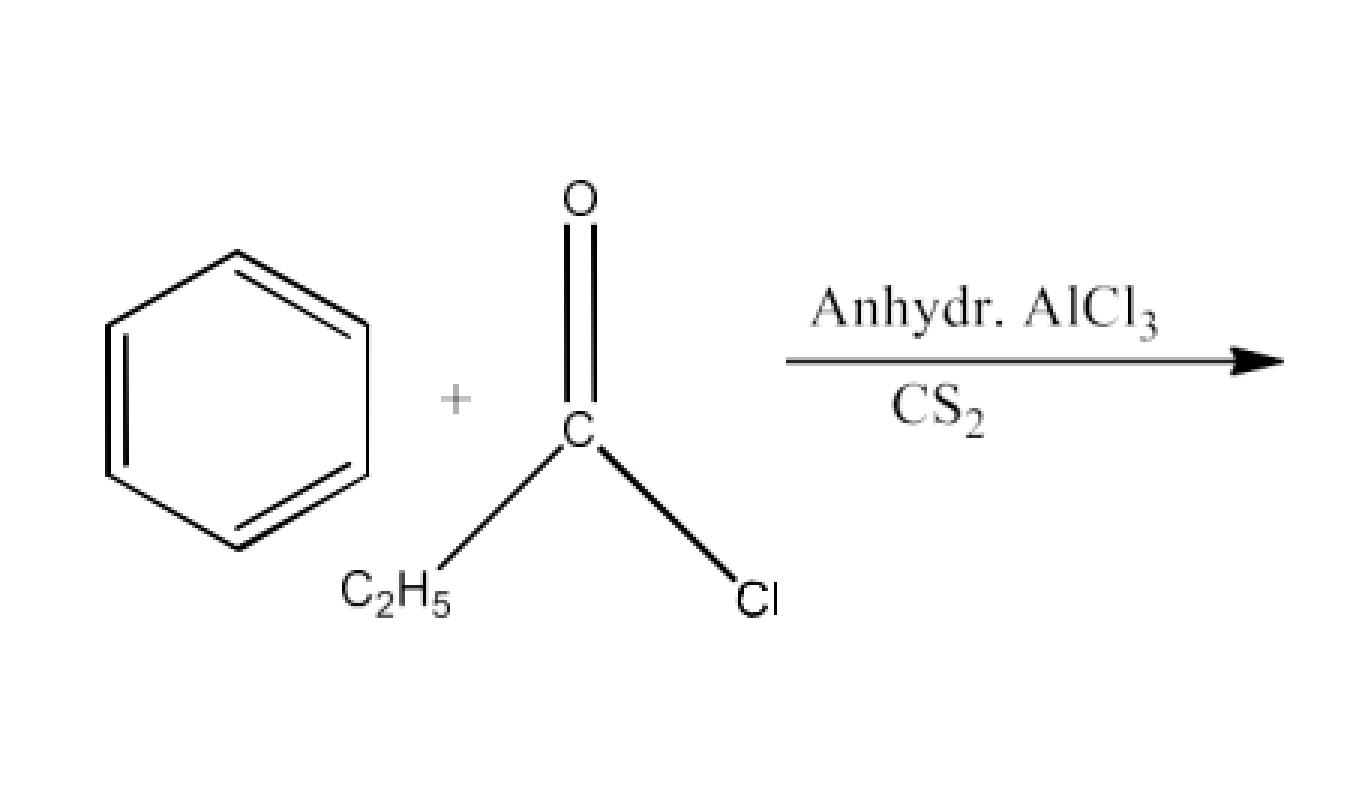
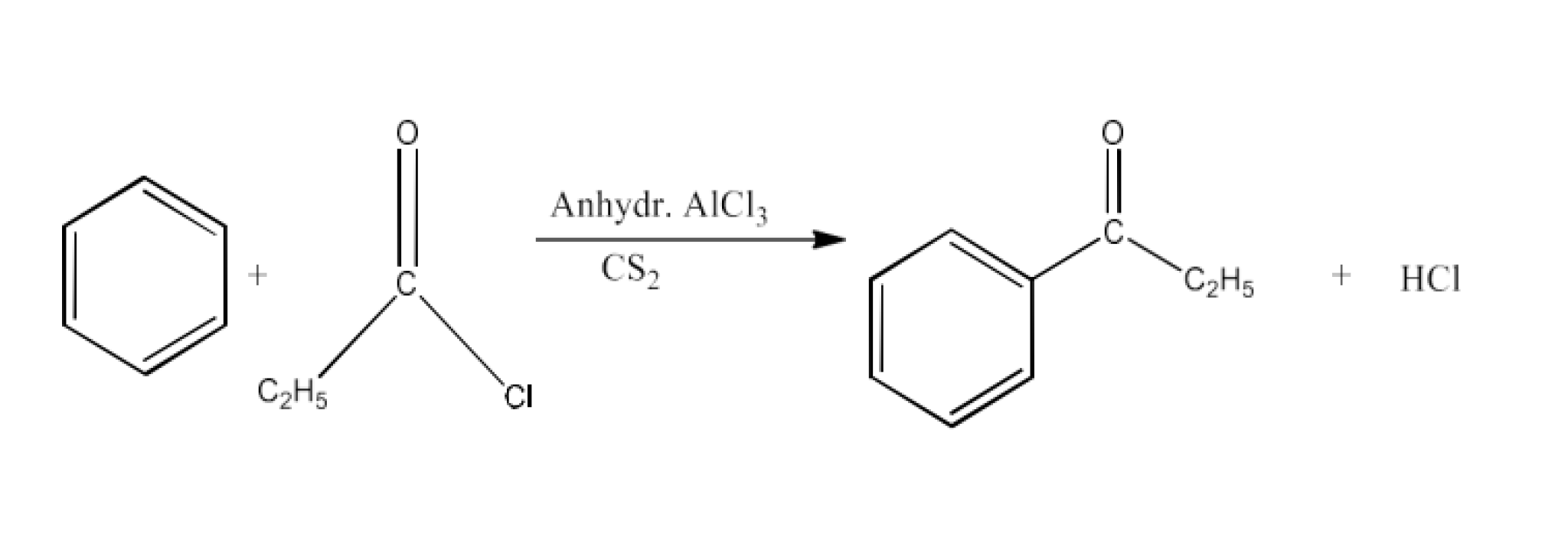
From acyl halides and anhydrides
From esters- Acidic hydrolysis of esters gives rise to carboxylic acids.
9. Why are aldehydes ketones and carboxylic acids important?
Aldehydes, ketones, and carboxylic acids are pivotal in organic chemistry, essential for biological processes and industrial synthesis, as emphasised in Class 12 Chemistry NCERT solutions.
10. What is the common name for ketones?
Ketones are commonly referred to as "oxo compounds," a recognized nomenclature detail stated in NCERT solutions for aldehydes ketones and carboxylic acids
11. Which has more effect: aldehyde or ketone?
Aldehydes generally exhibit stronger effects than ketones, a comparison drawn in NCERT solutions for aldehydes and ketones in Class 12 Chemistry.
12. What happens when you add an acid to an aldehyde?
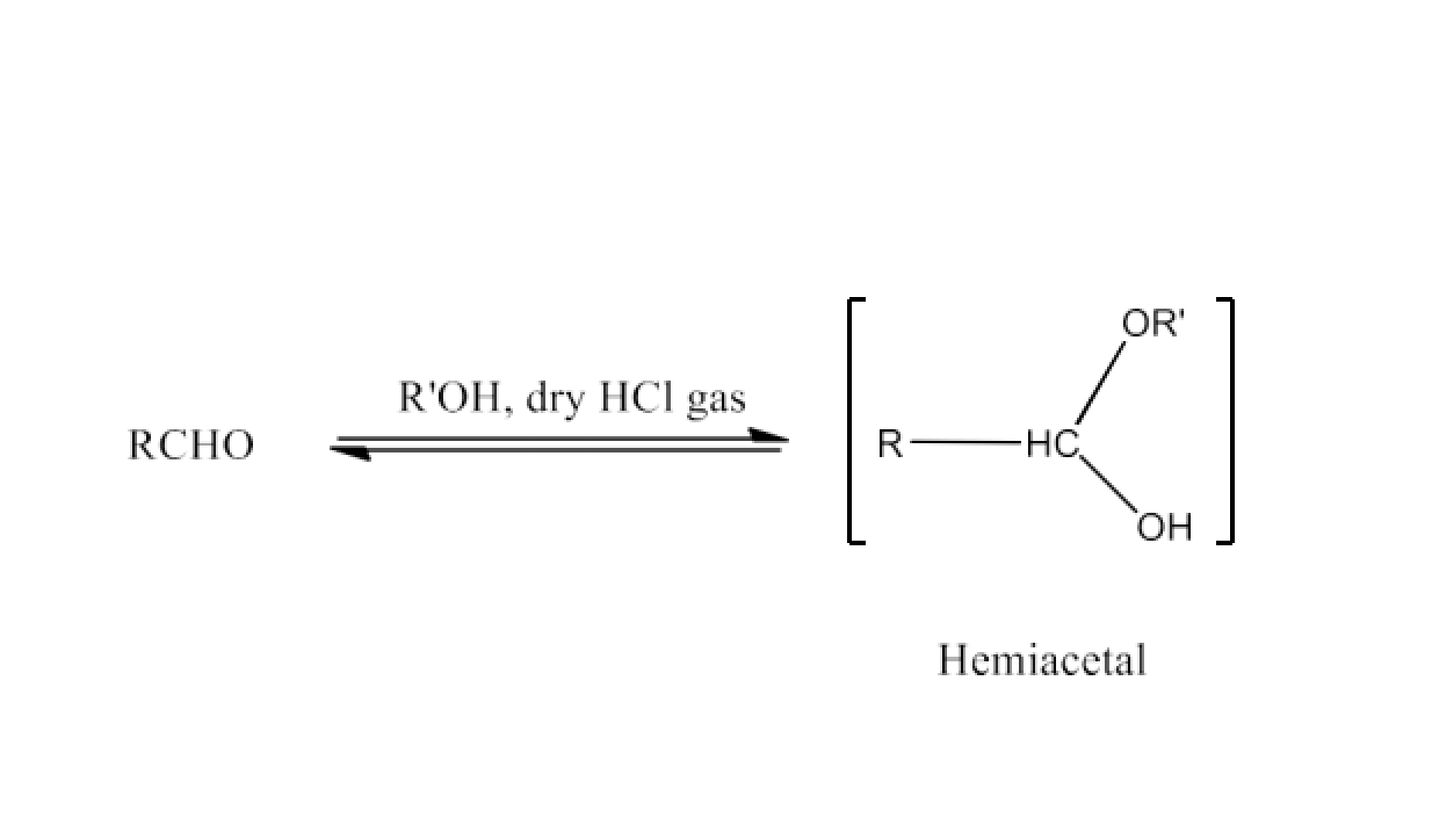
Adding an acid to an aldehyde leads to the formation of an acetal or hemiacetal, a chemical transformation highlighted in aldehyde and ketone NCERT solutions.
13. Why is carboxylic acid stronger than aldehyde?
Carboxylic acids surpass aldehydes in acidity due to the additional -COOH group, a key concept elucidated in aldehyde ketone and carboxylic acids NCERT solutions.
14. What are 3 uses of aldehydes and ketones?
Aldehydes and ketones serve as solvents, preservatives, and intermediates in pharmaceuticals and perfumes, as outlined in NCERT solutions for aldehydes, ketones, and carboxylic acids in Class 12 Chemistry.