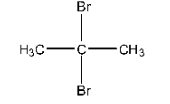
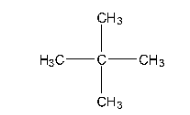
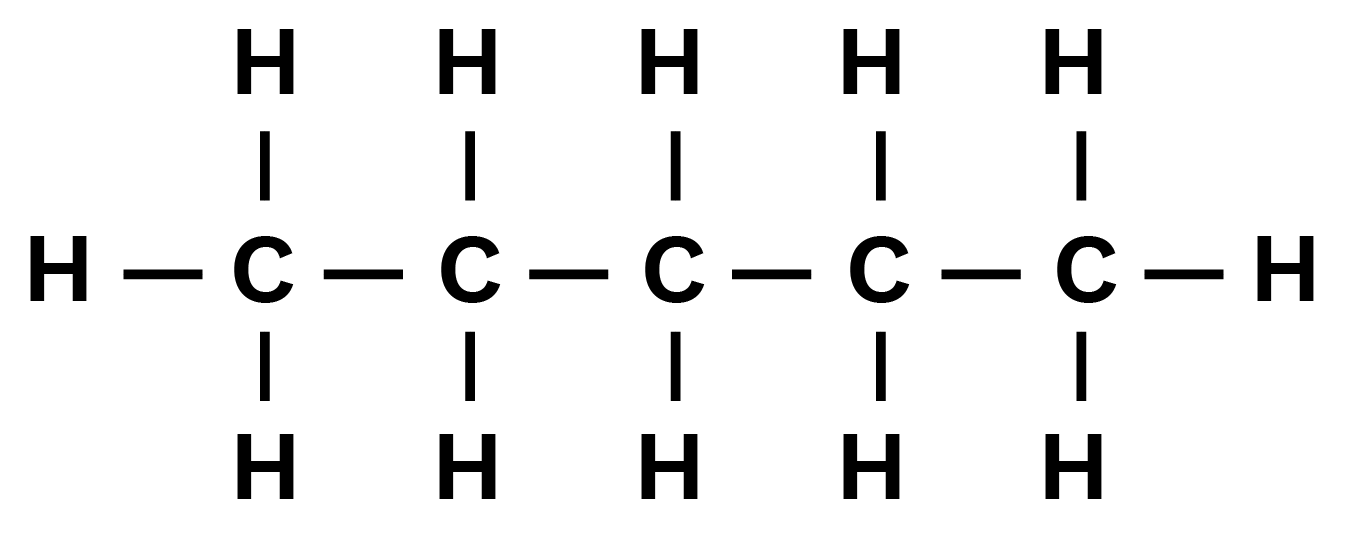
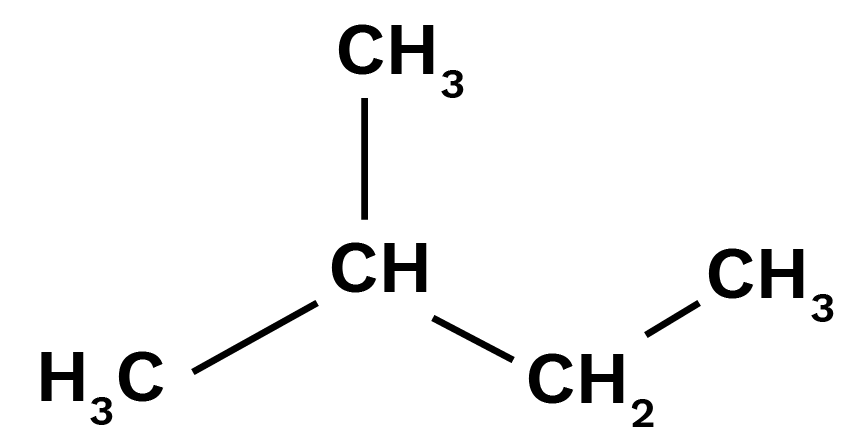
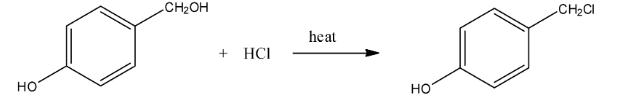
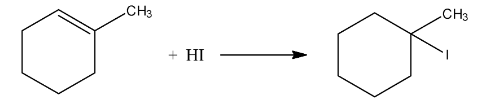
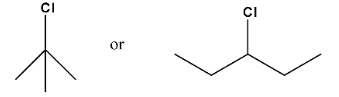
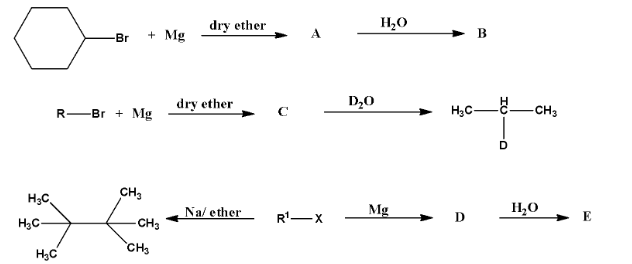
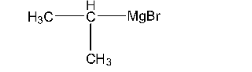
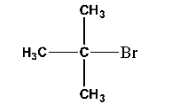
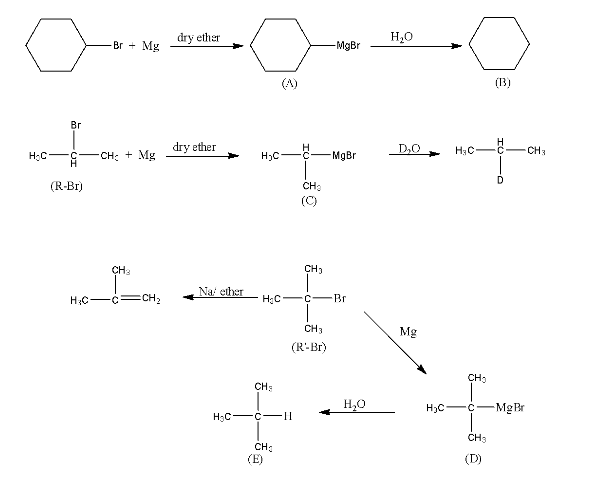
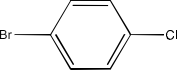
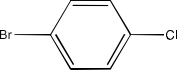
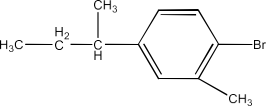
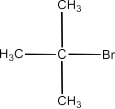
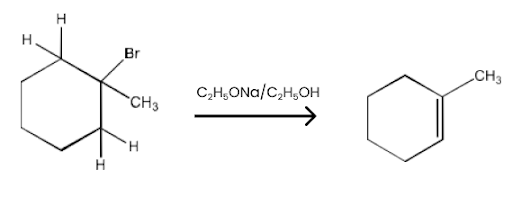
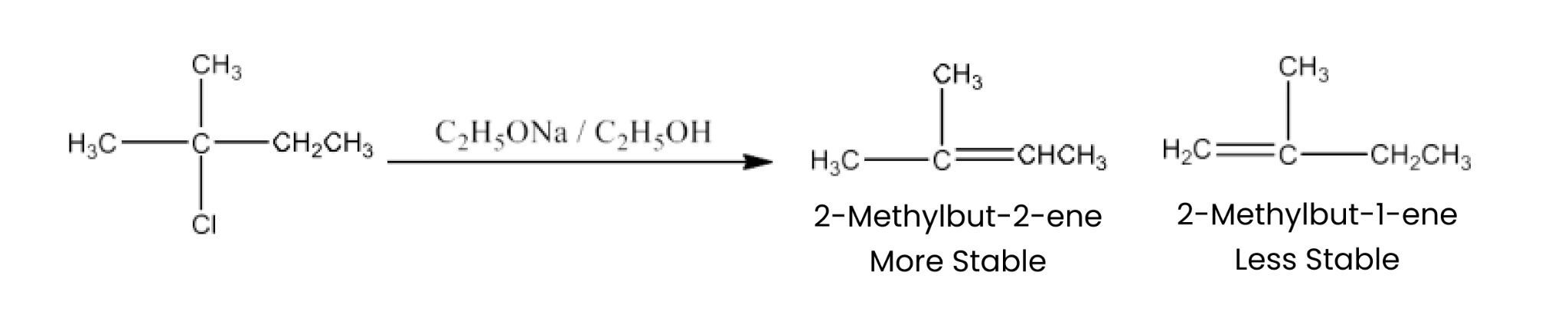
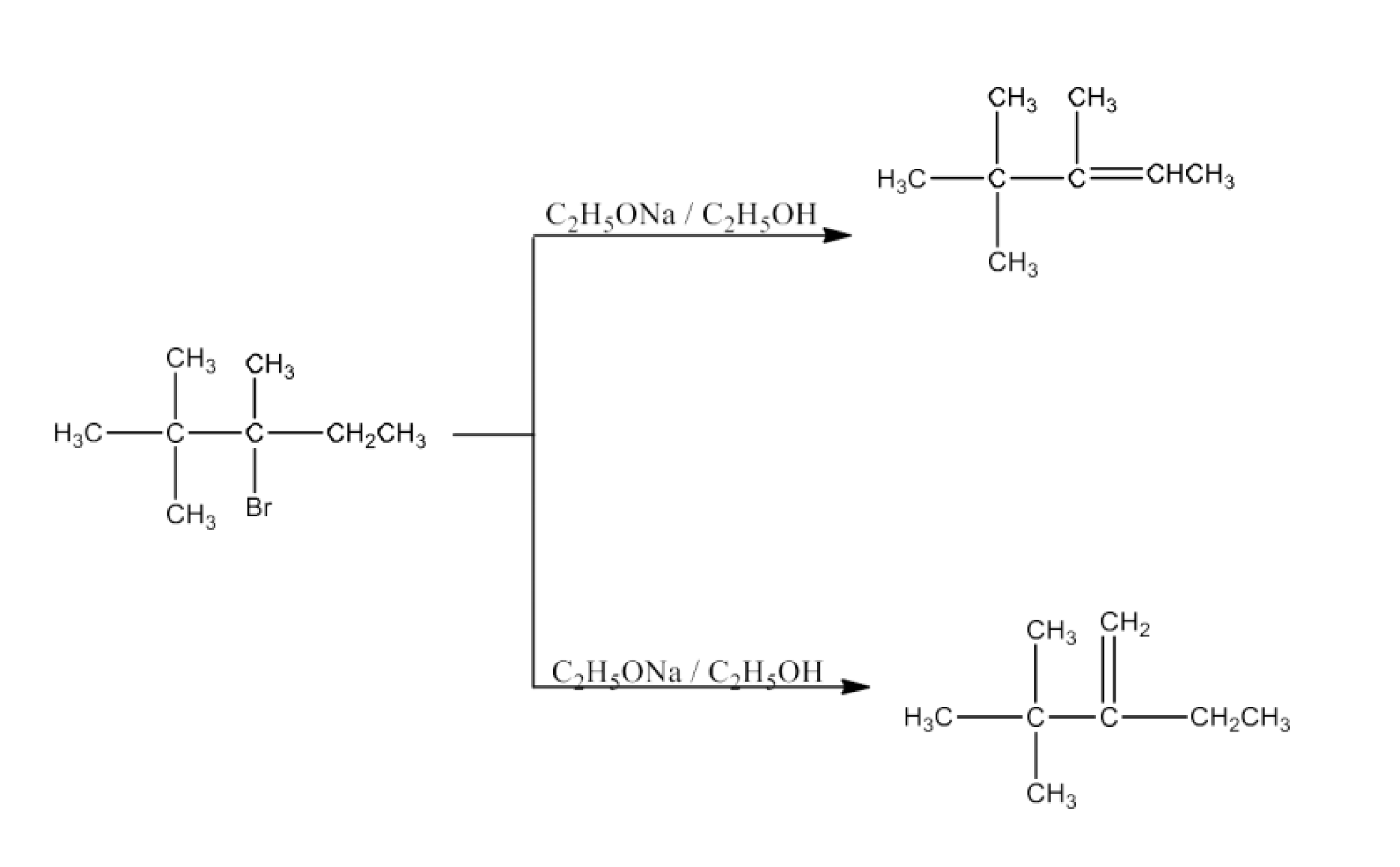
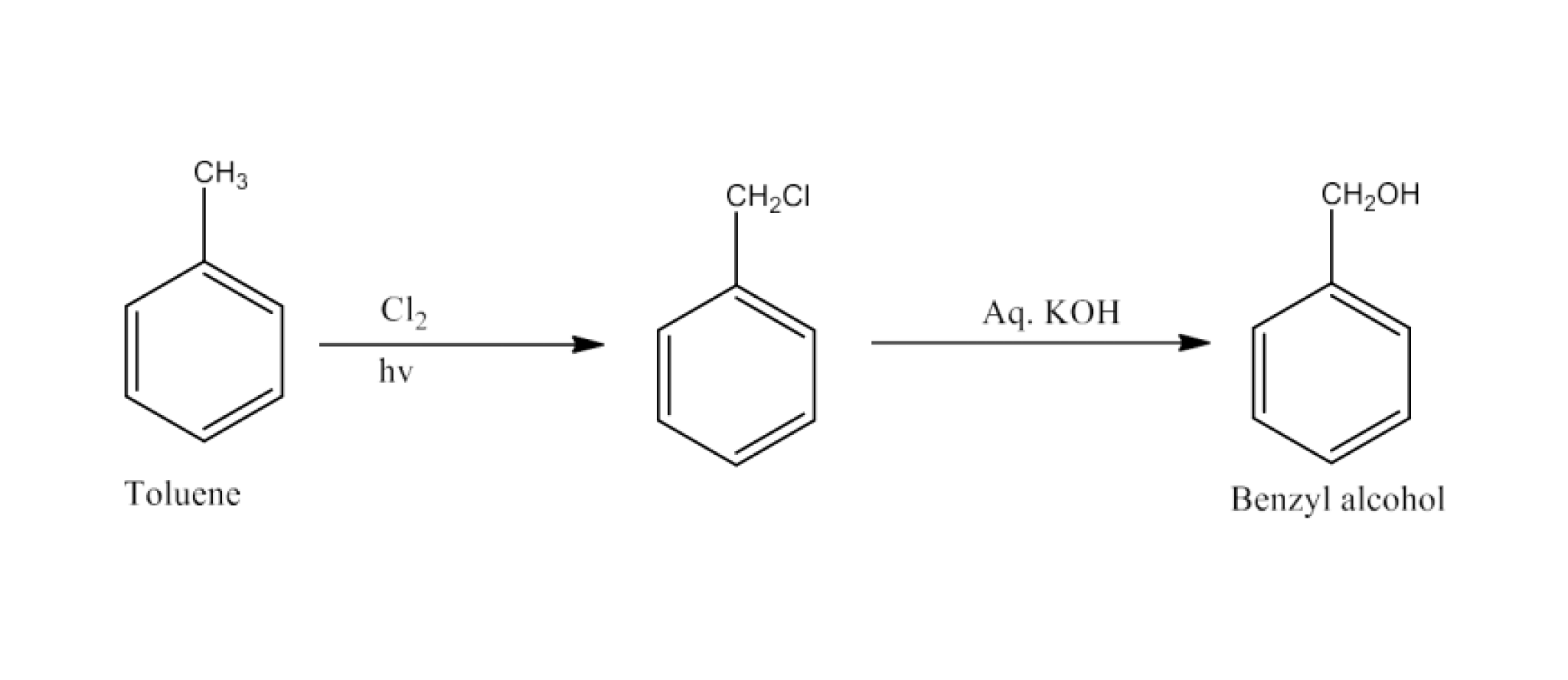
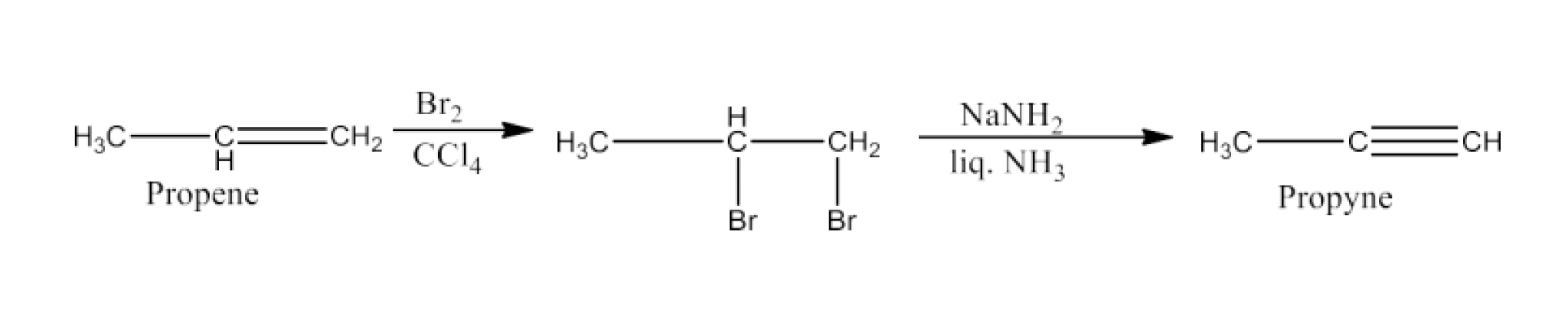
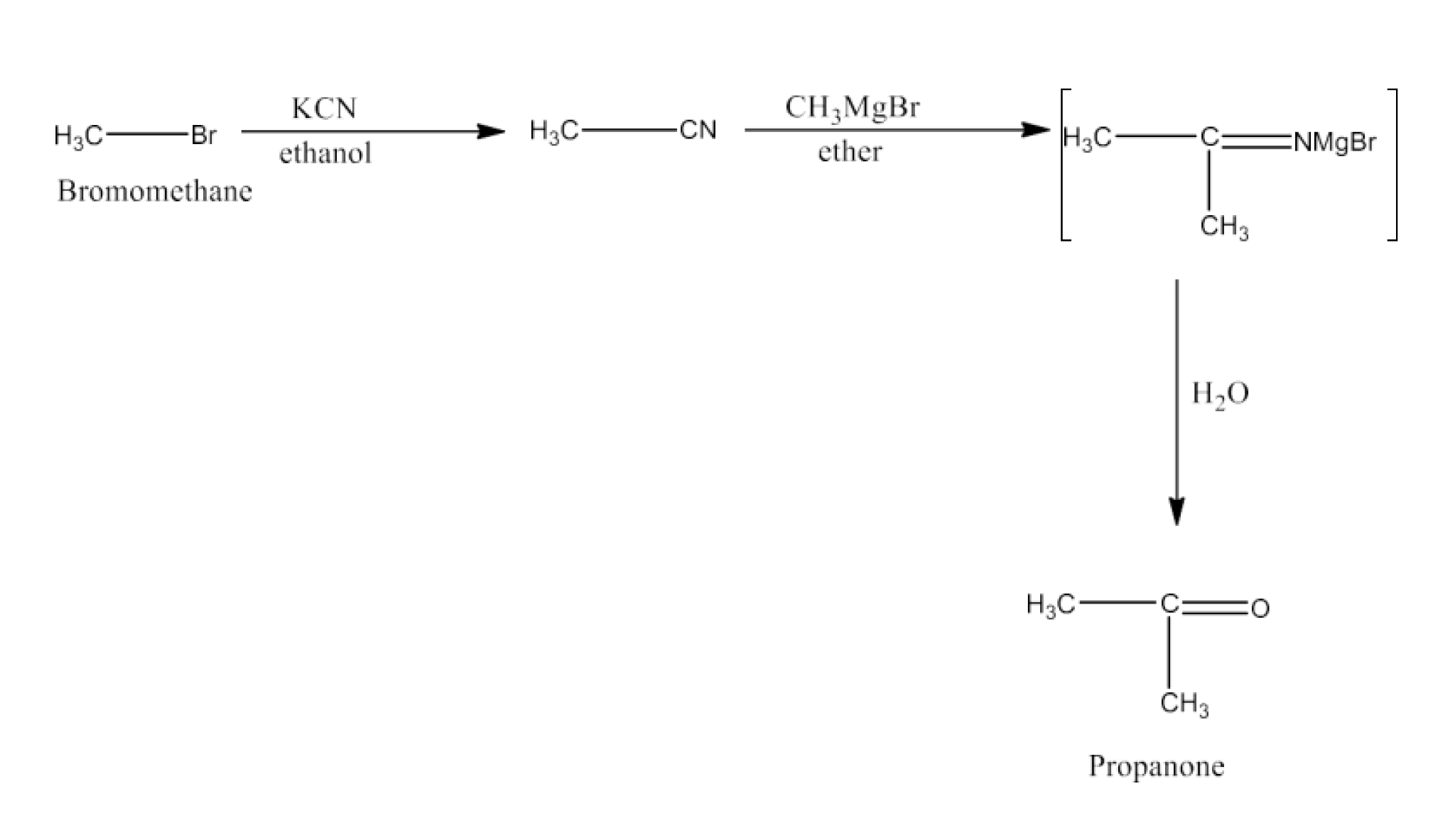
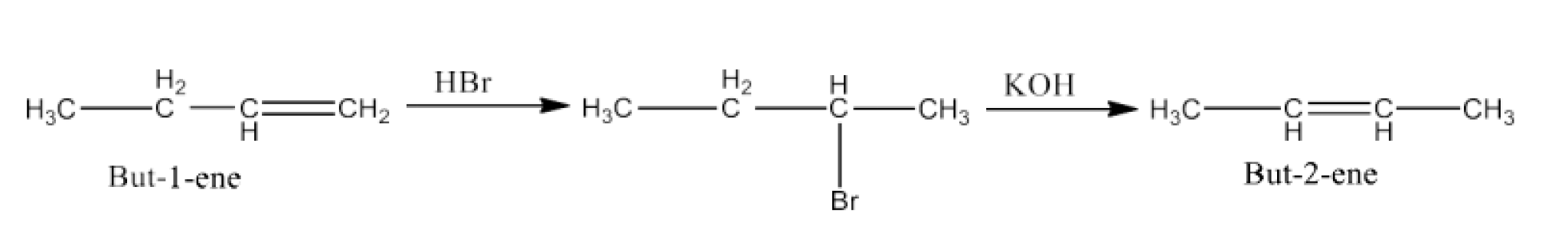
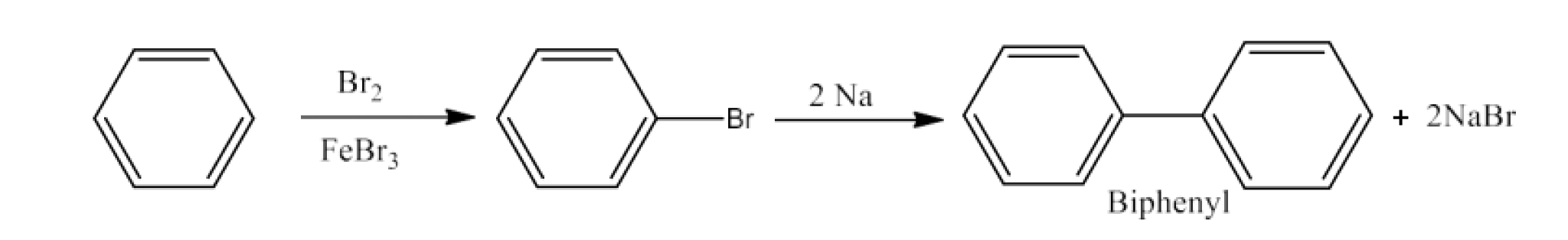
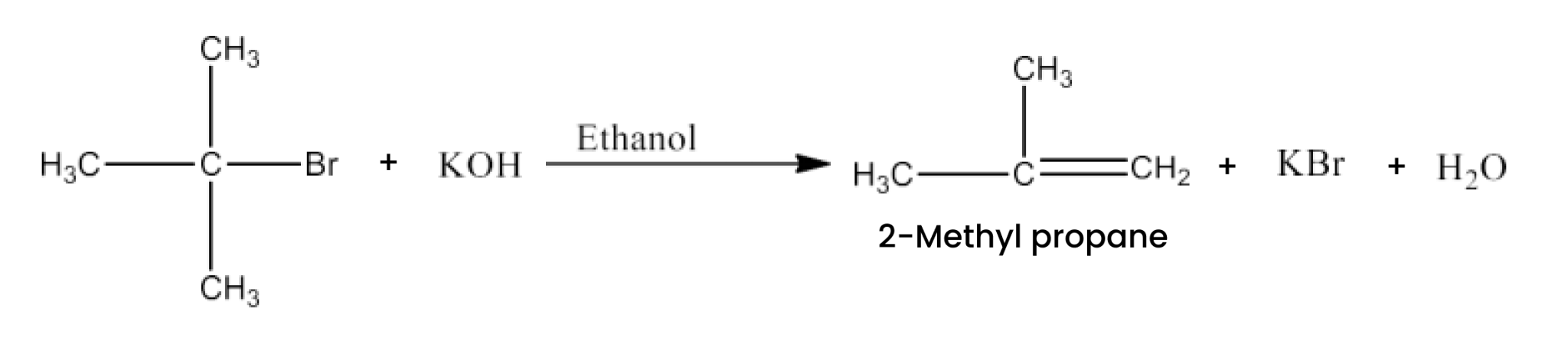
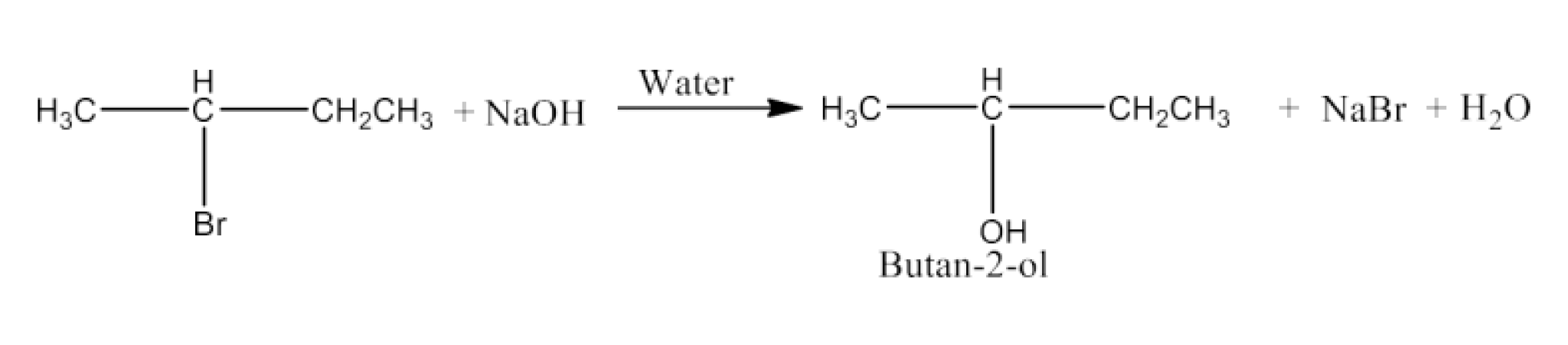
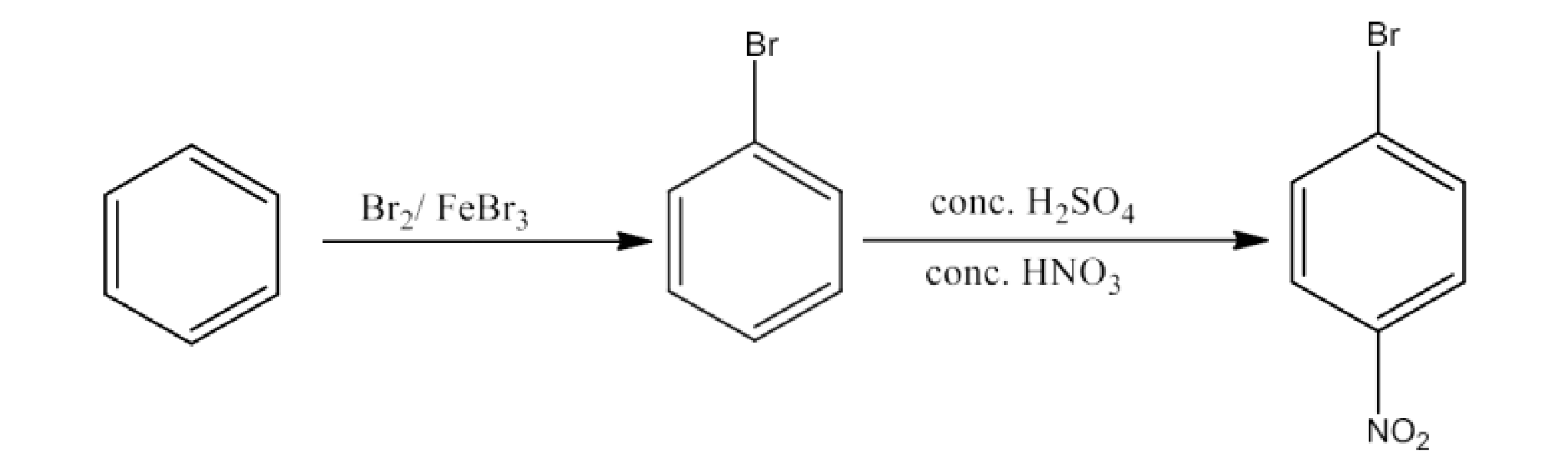
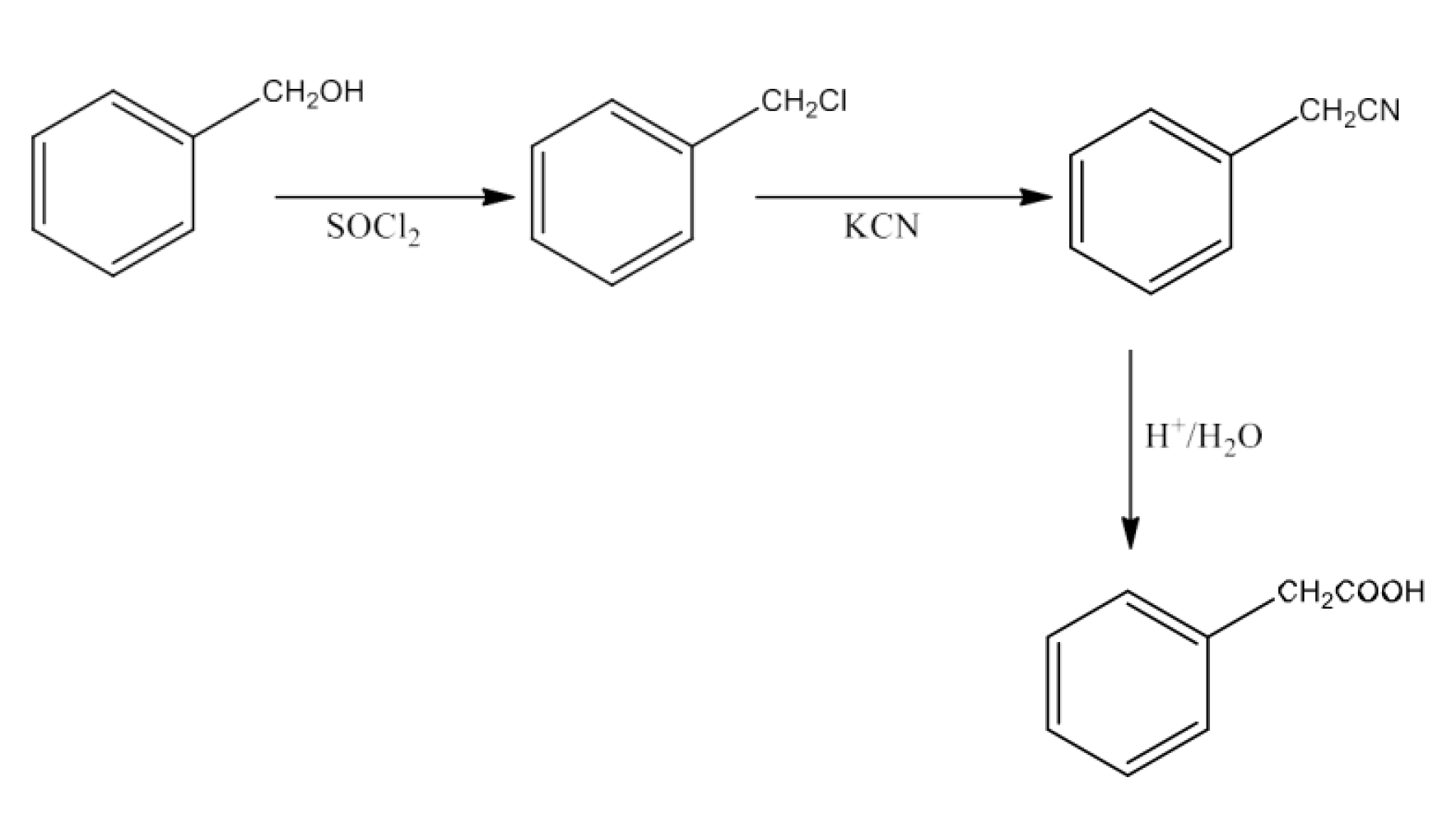
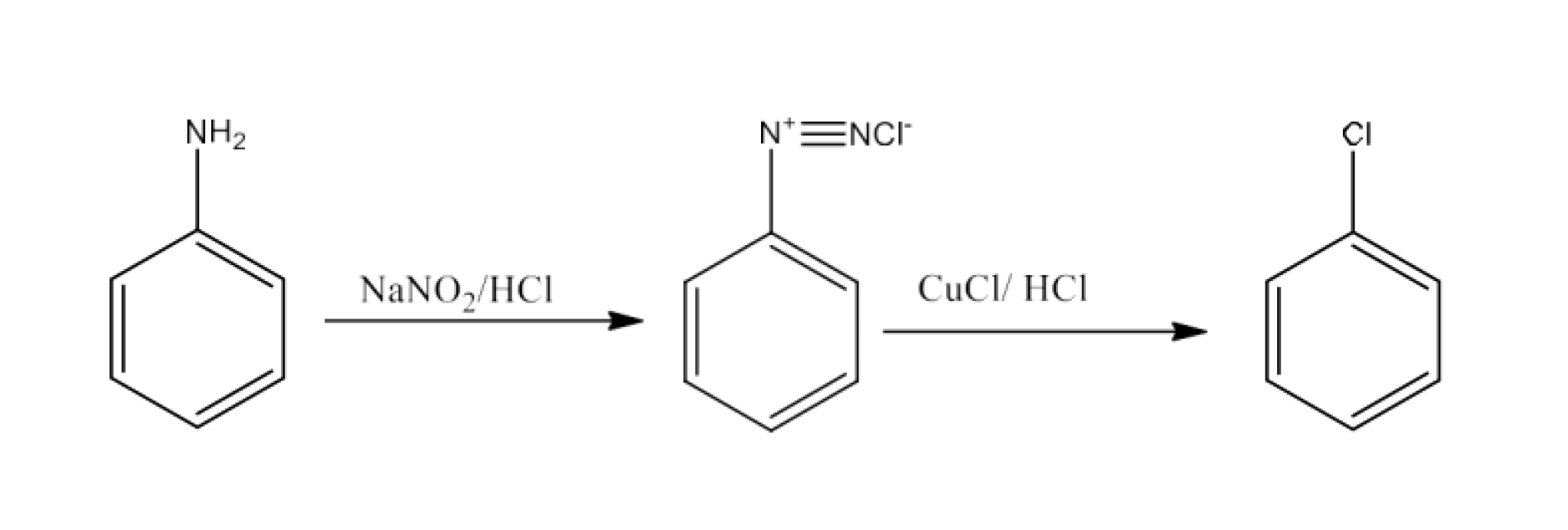
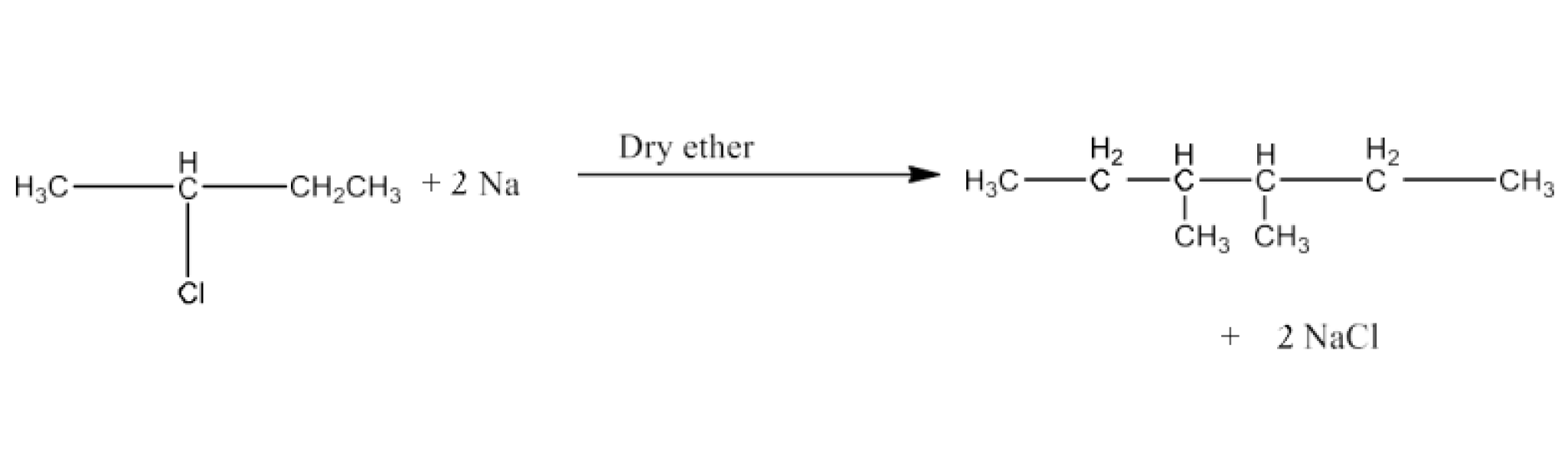
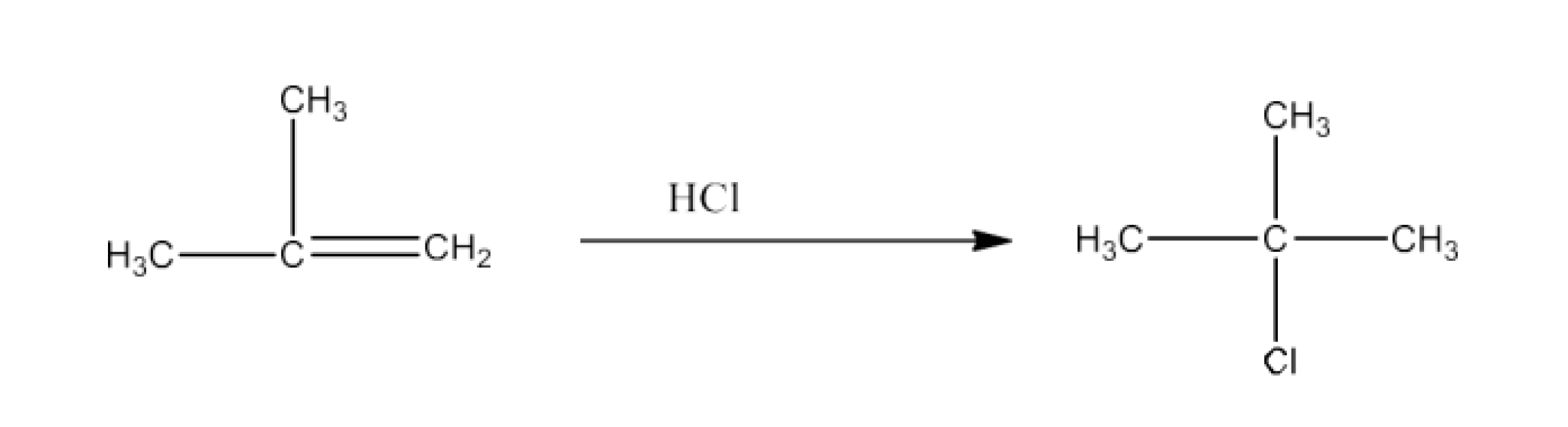
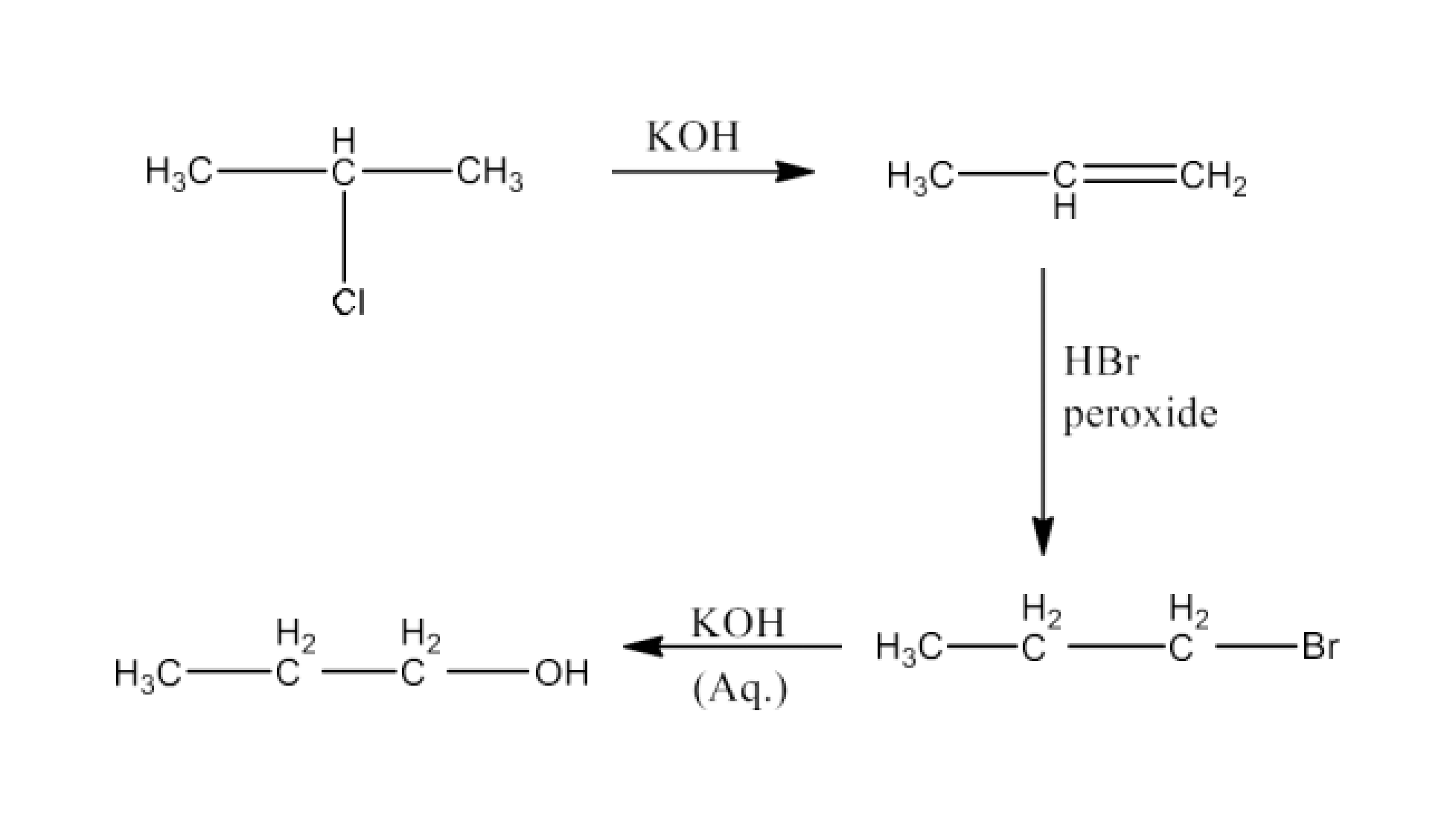
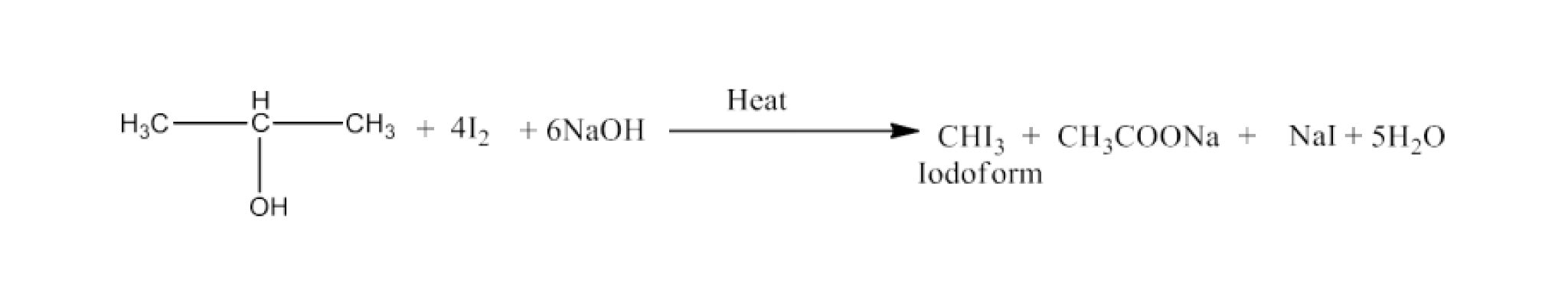
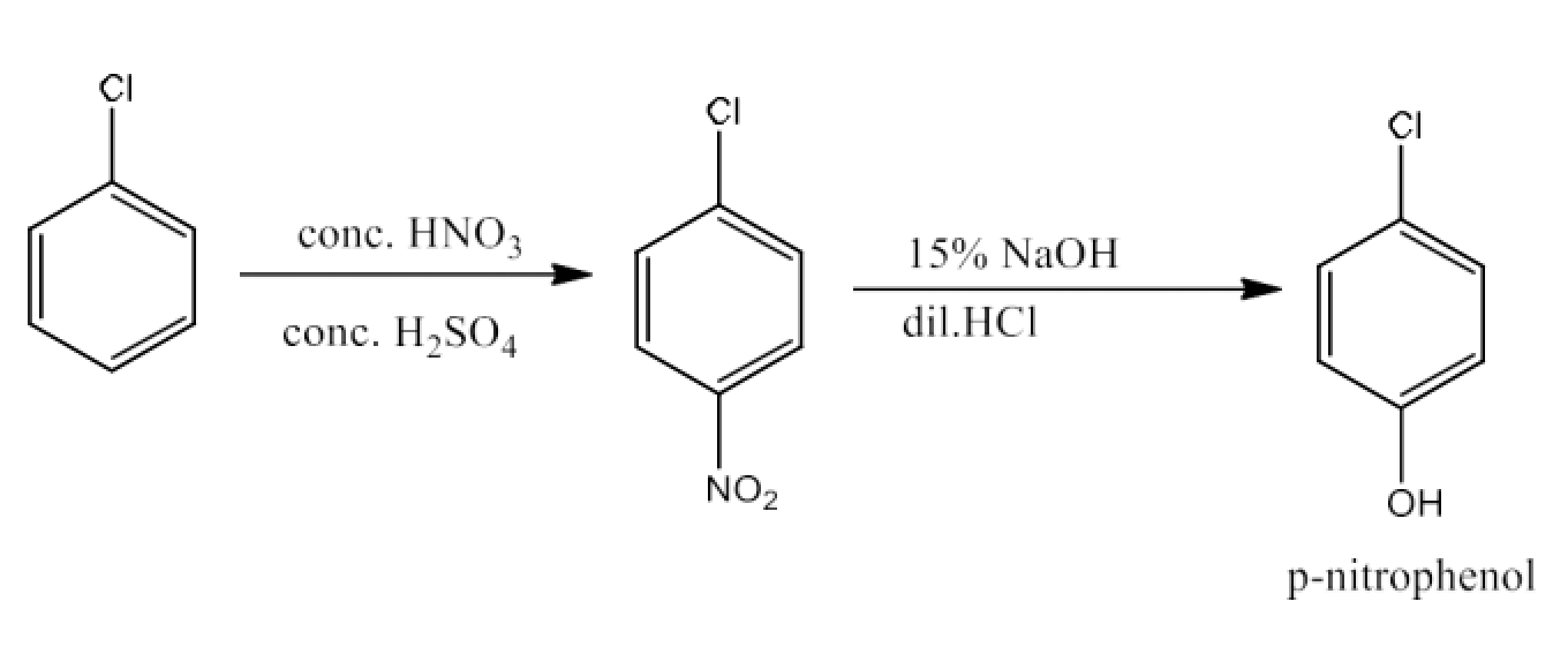
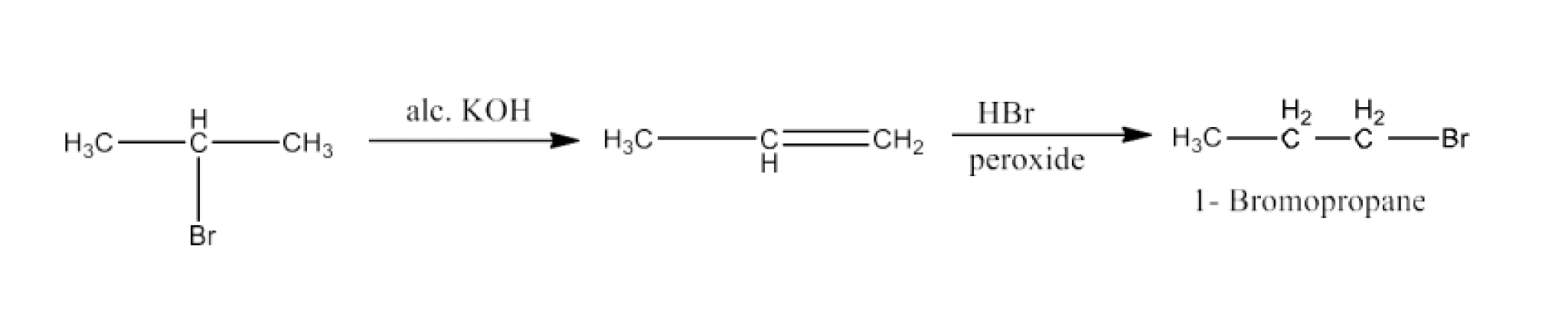
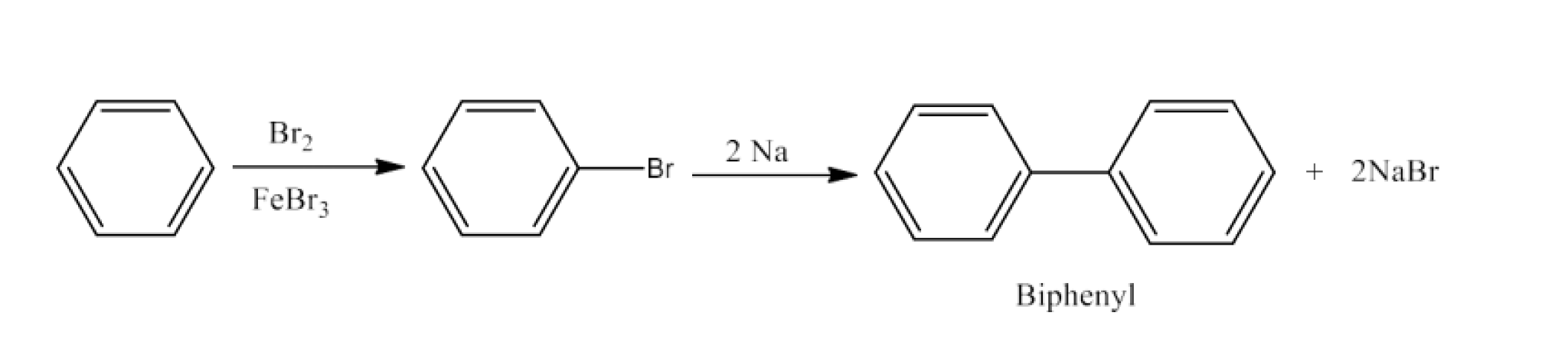
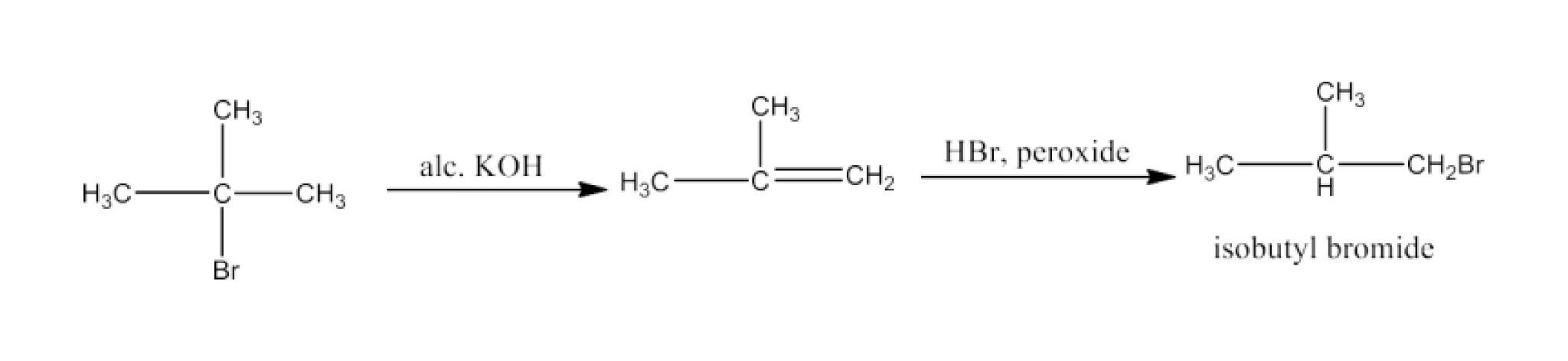
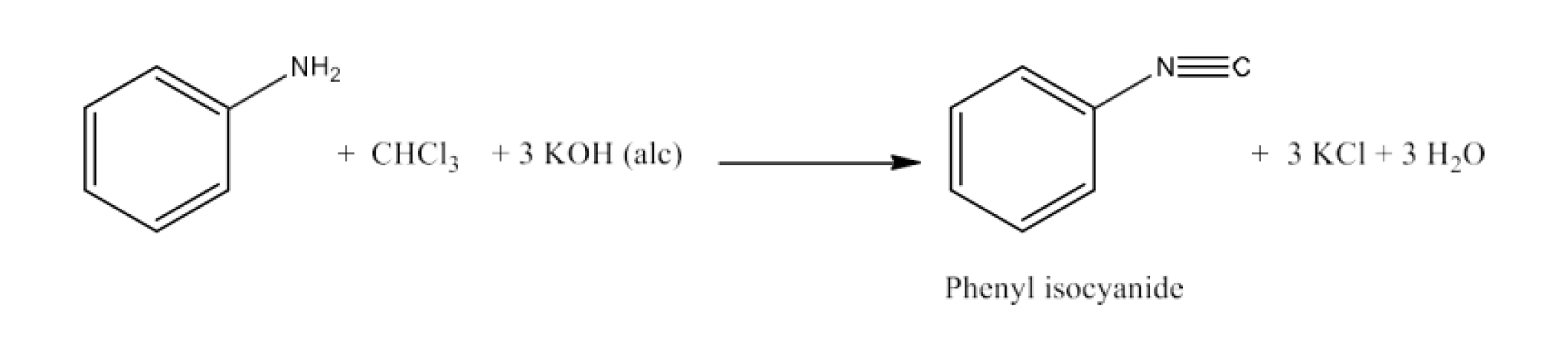
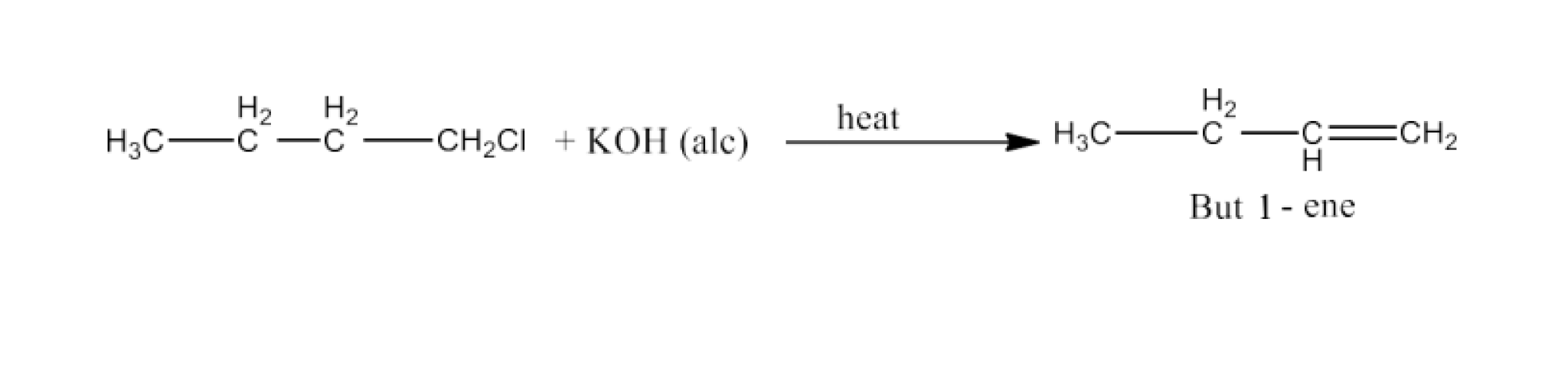
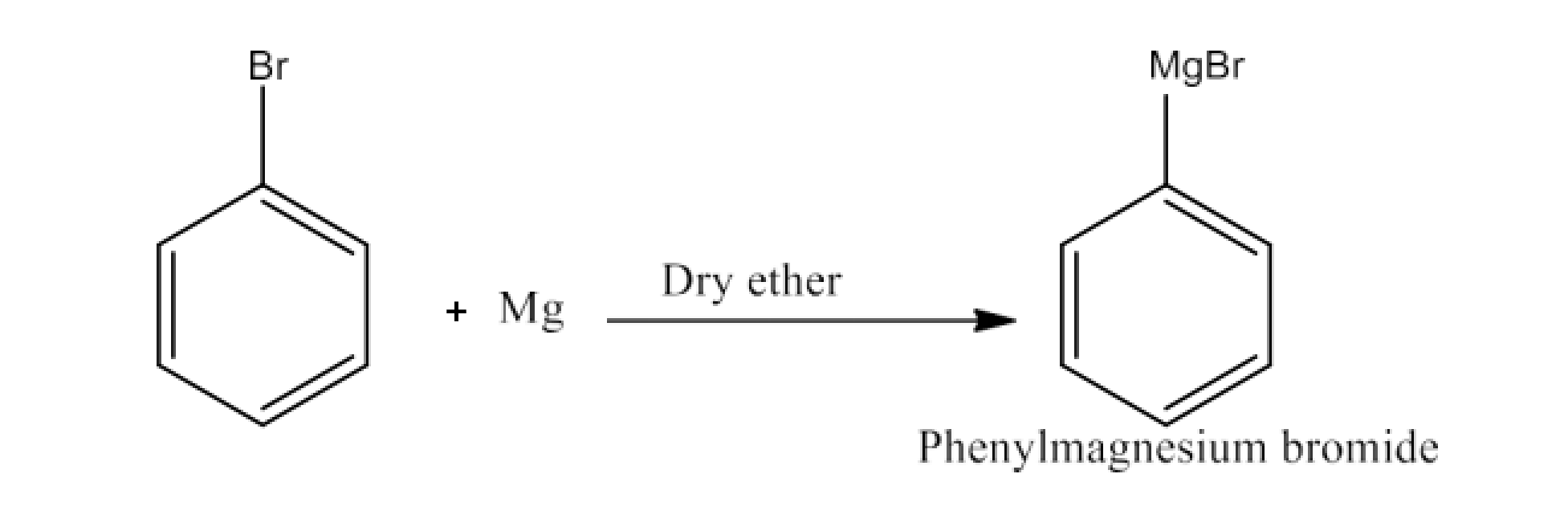
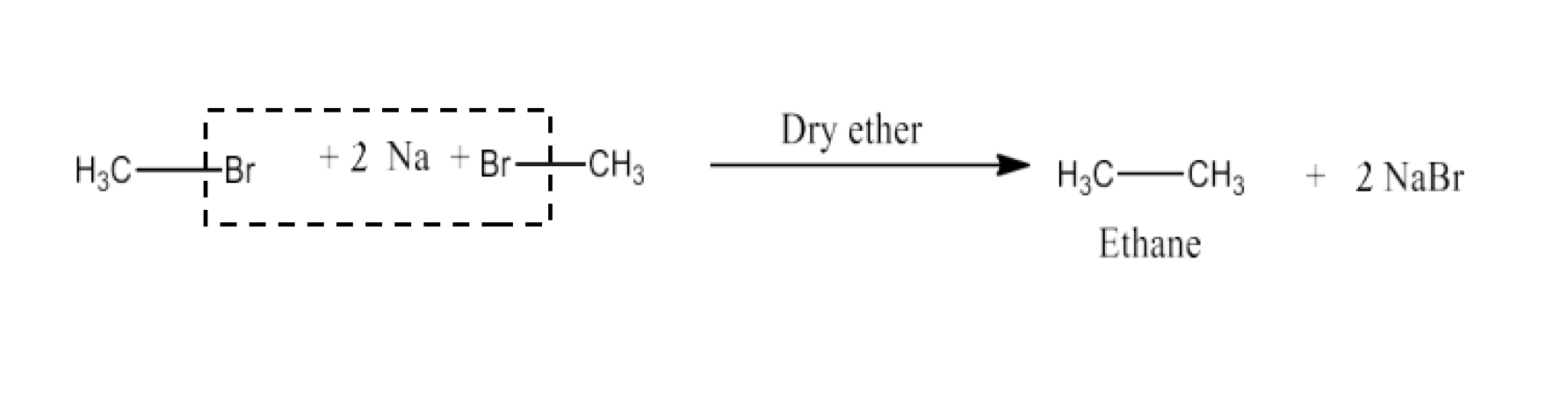
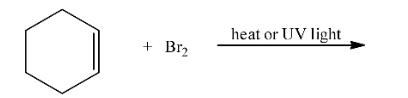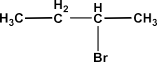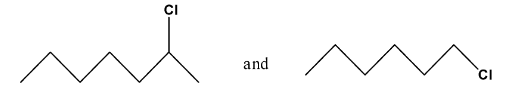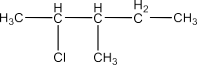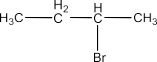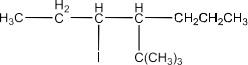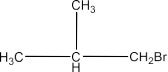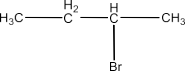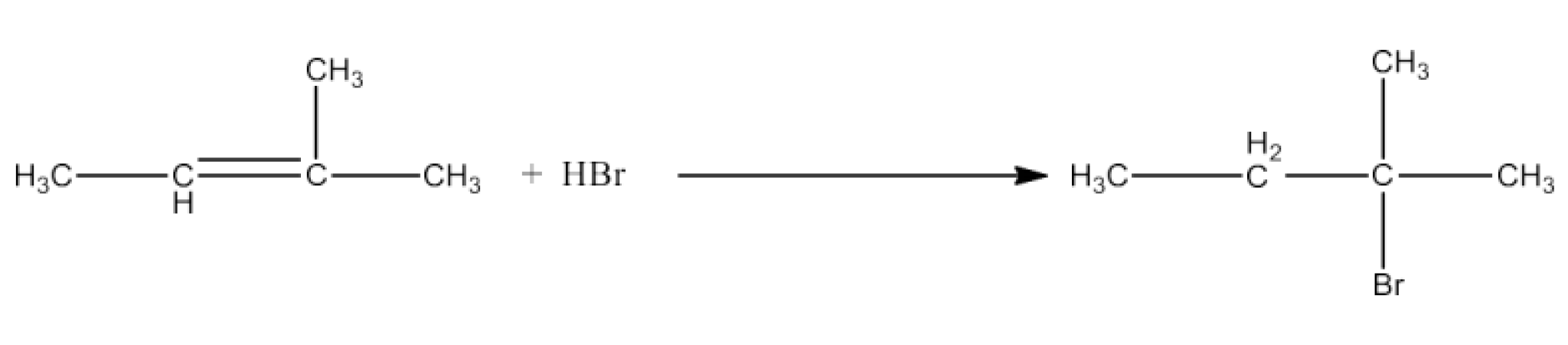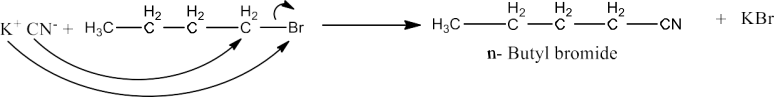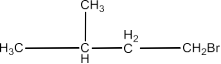Haloalkanes and Haloarenes Class 12 NCERT Solutions FREE PDF Download




























FAQs on NCERT Solutions for Class 12 Chemistry Chapter - 6 Haloalkanes and Haloarenes
1. Why Should I Refer to NCERT Solutions for Class 12 Chemistry Haloalkanes and Haloarenes PDF?
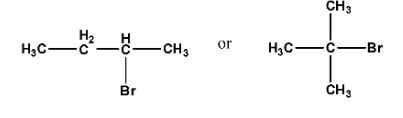
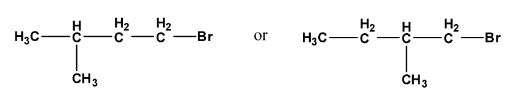
NCERT solutions for class 12 chemistry haloalkanes and haloarenes pdf deals with definitions, classifications, examples, and properties of halogen compounds. The solution provides an extensive study of physical and chemical properties, the various methods of preparation, and the usage of organo-halogen compounds.
By going through these NCERT solutions in PDF format, students will be able to name Haloalkanes and Haloarenes according to the IUPAC system of nomenclature from their provided structures. Also, students will get to learn different structures of major organic products in various reactions, mechanisms of a given reaction, usage of DDT, carbon tetrachloride, and so on. In short, the detailed discussion of the NCERT solutions helps students to understand the concept better.
2. How Does Haloalkanes and Haloarenes Class 12 NCERT Solutions Help Students?
NCERT Solutions Class 12 Chemistry Haloalkanes and Haloarenes revolves around the important methods of preparation, uses, and physical and chemical properties of organo-halogen compounds. Besides, each of the NCERT solutions is prepared by following the latest CBSE Board guidelines. The solutions help students to better understand the concept and score well in the 12th boards and other competitive examinations.
3. What are the Subtopics of the Chapter Haloalkanes and Haloarenes?
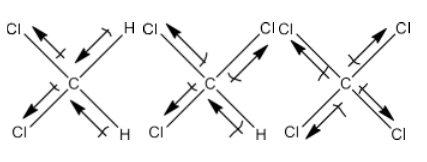
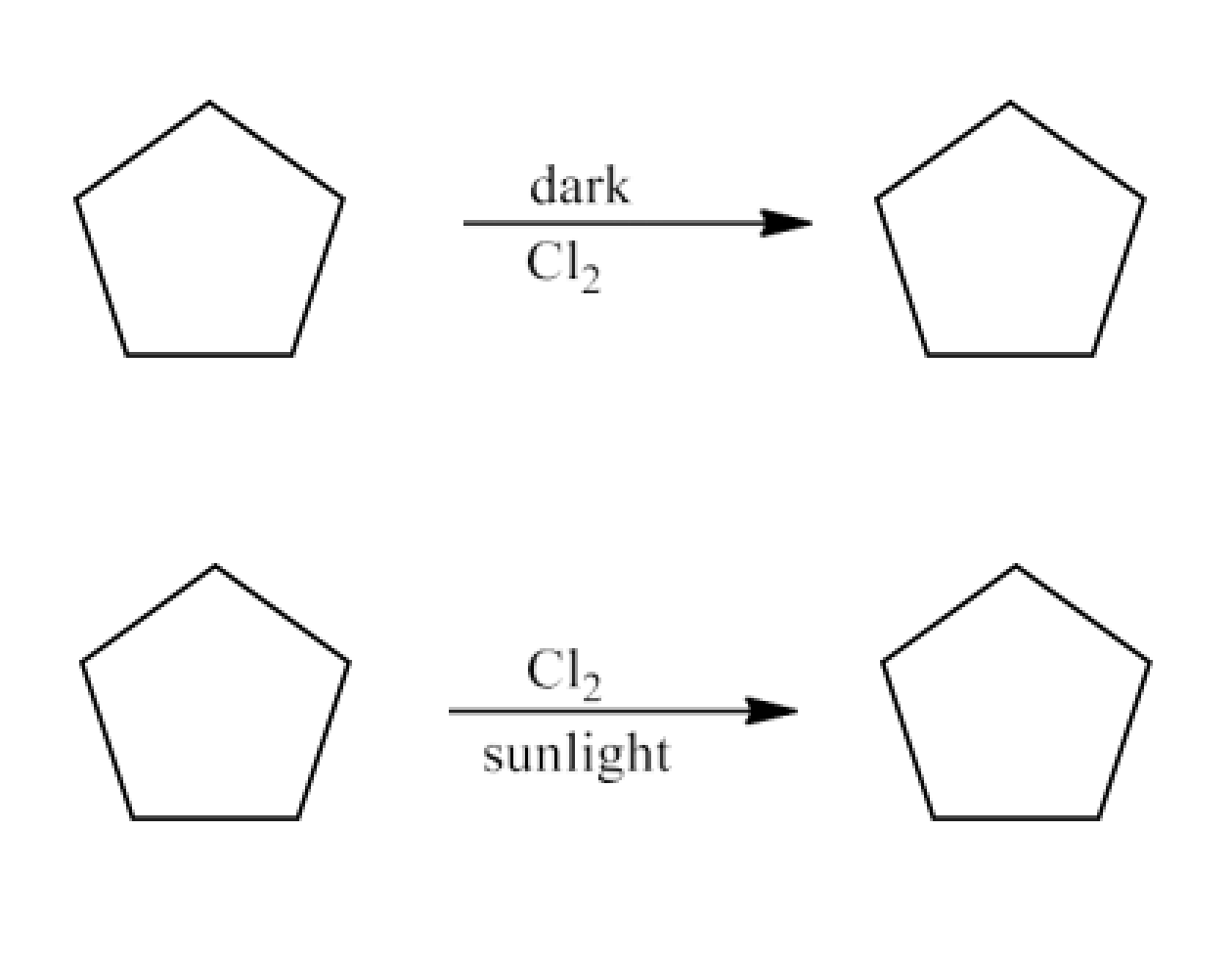
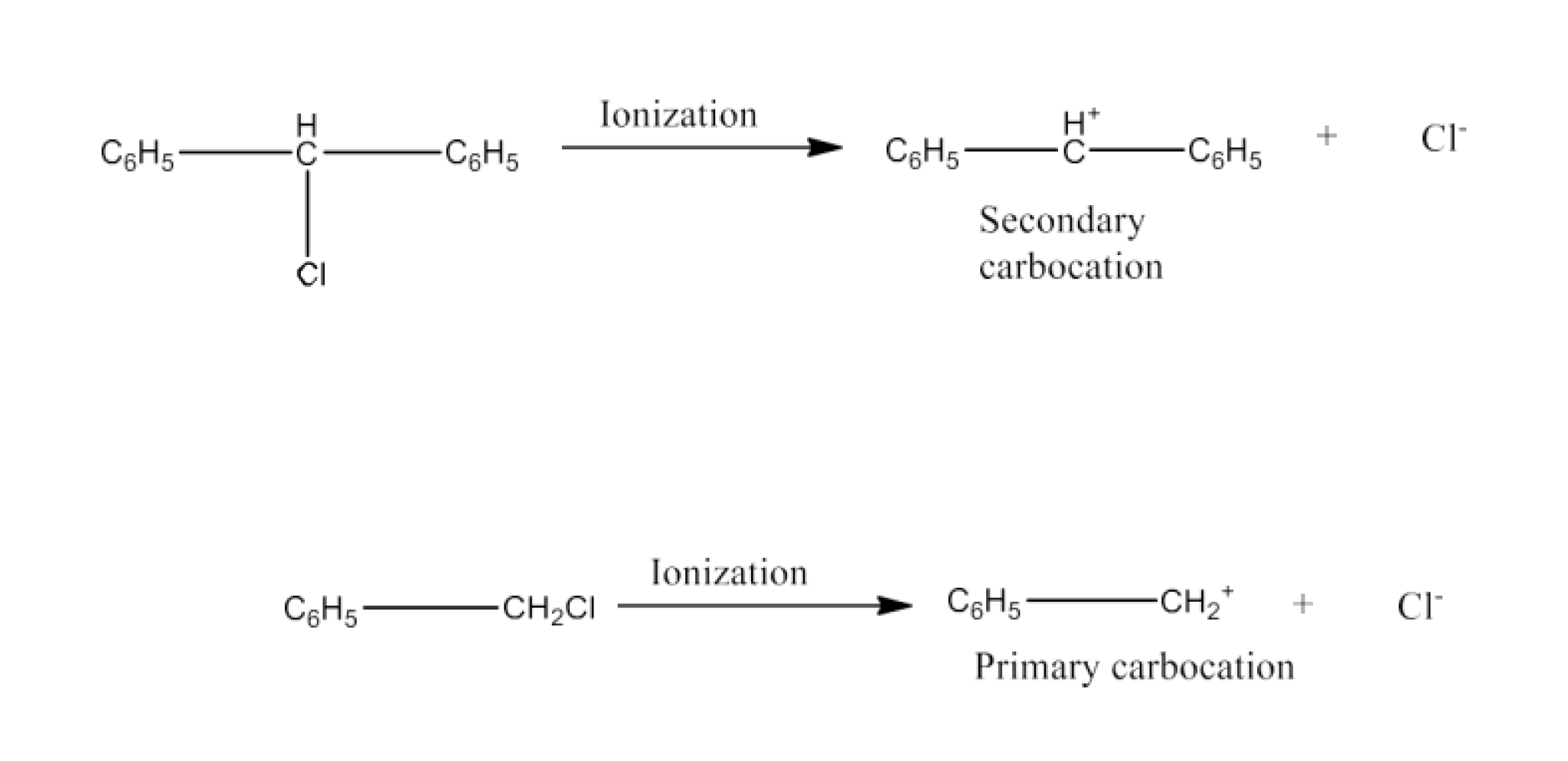
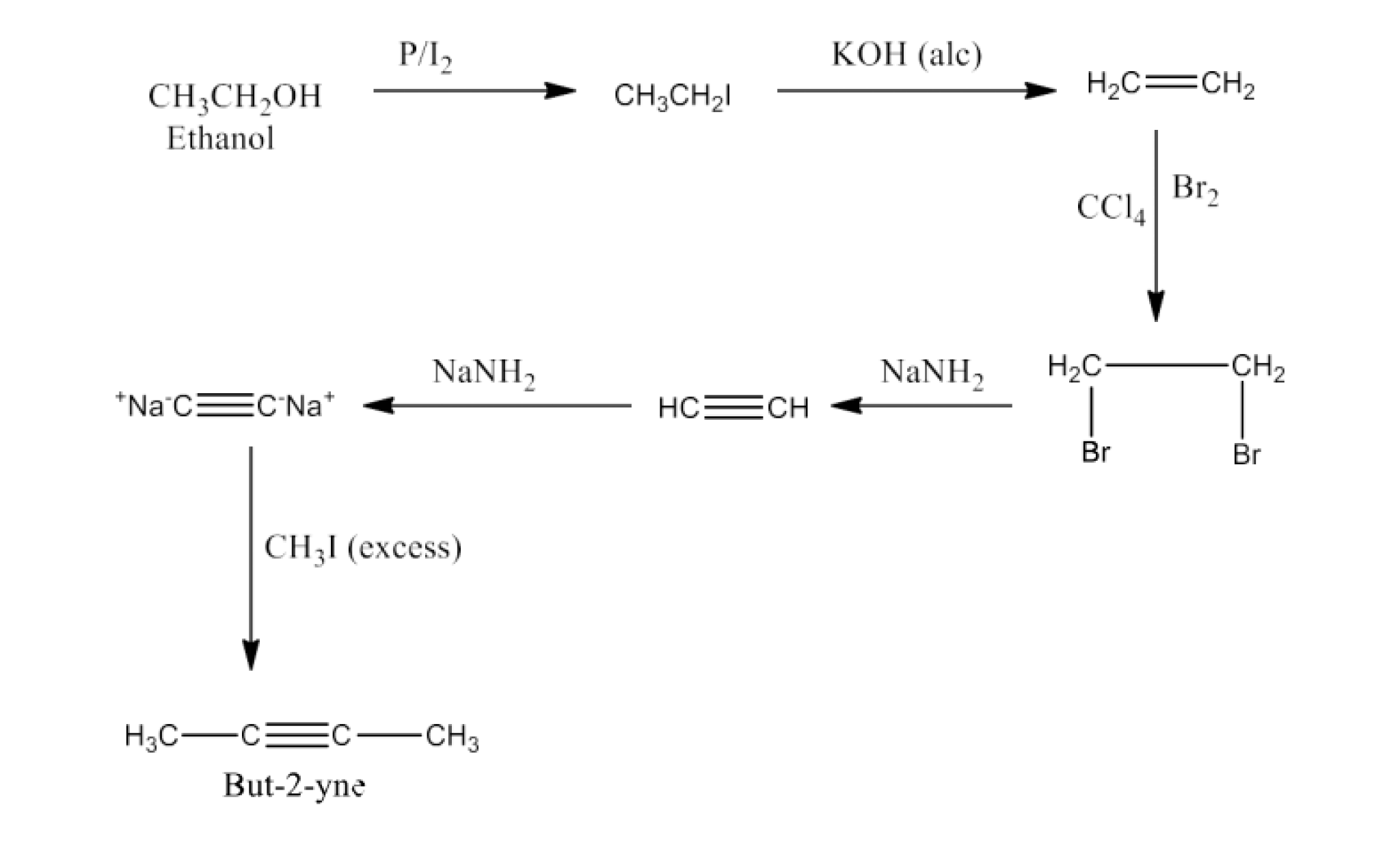
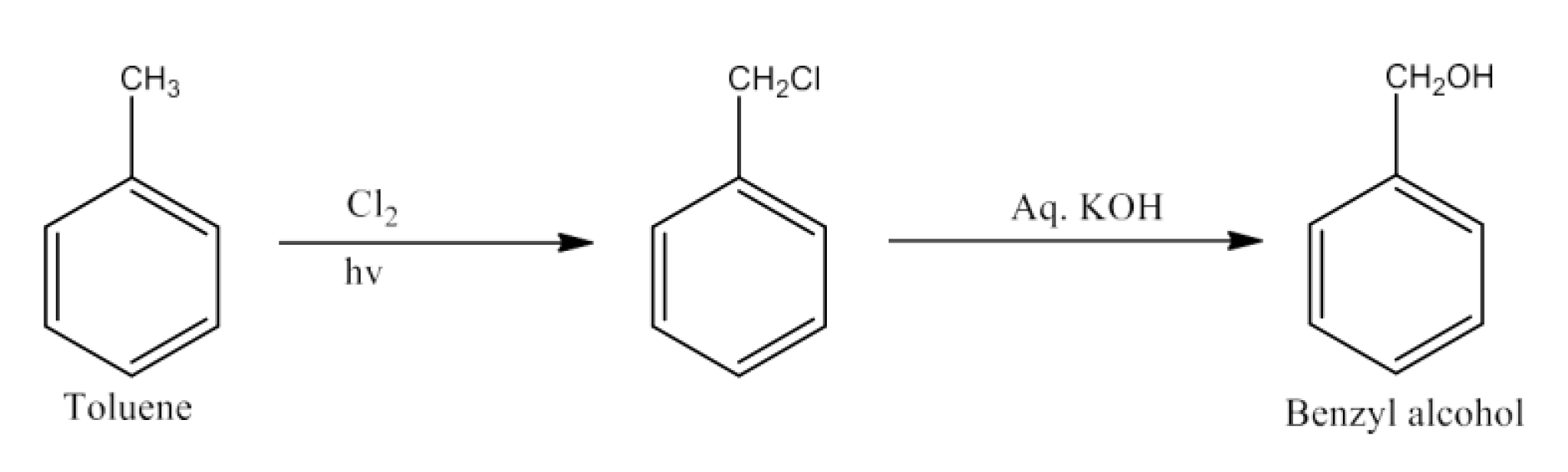
The chapter of Haloalkanes and Haloarenes consists of several sub-topics. They are – (1) Classification, (2) Nomenclature, (3) Nature of C-X Bond, (4) Methods of Preparation, (5) Physical properties, (6) Chemical Reactions, (7) Polyhalogen Compounds.
Students who intend to not only ace in the CBSE 12th board examinations but also in competitive exams like JEE can refer to these extensive study materials to get a better understanding of the topics covered in this chapter. Likewise, students who are finding it difficult to understand the underlying concept and sub-topics of Haloalkanes and Haloarenes may refer to various other related study materials on Vedantu, for a good exam preparation.
4. How do the NCERT Solutions for Chapter 6 of Class 12 Chemistry PDF help students?
Chapter 6 of Class 12 Chemistry deals with the preparation methods, applications, chemical and physical properties of Haloalkanes and Haloarenes. The NCERT Solutions are framed by the faculty having vast experience at Vedantu as per the latest CBSE guidelines and exam patterns. By referring to these solutions, students can easily understand the concepts better and score well in the board exams. They can also download the PDF format of solutions and can be used to check if their answers are right or wrong while answering the questions from the NCERT textbook. The solutions are available free of cost on the Vedantu website and the Vedantu app. You can download class 12 chemistry chapter haloalkanes and haloarenes NCERT solutions PDF for the detailed study.
5. Explain why alkyl halides, though polar, are immiscible (not soluble) with water, according to Chapter 6 of Class 12 Chemistry?
Alkyl halides are polar molecules. That is the reason why their molecules are held together by dipole-dipole forces. Whereas the molecules of H20 are held together by the hydrogen bonds. So, when alkyl halides are added into water, the new forces of attraction between water and haloalkane molecules becomes weaker than the forces of attraction existing between alkyl halide-alkyl halide molecules and water-water molecules. Hence, alkyl halides, even though polar, are not soluble in water.
6. How to answer difficult questions of class 12 chemistry chapter haloalkanes and haloarenes NCERT solutions?
To answer the difficult questions of class 12 chemistry chapter haloalkanes and haloarenes NCERT solutions students must be well prepared with all the basic concepts. It is because having a strong knowledge of the fundamental concepts will help the students to answer any difficult questions. Students should give more importance to the topics that have more weightage and those important from the exam point of view. They should clear all their doubts regarding any chapter and have a clear knowledge of that full chapter.
7. Can I score high marks using the NCERT Solutions for Chapter 6 of Class 12 Chemistry?
Students can score high marks using the NCERT Solutions for Chapter 6 of Class 12 Chemistry as it guides them to prepare for the exams without fear. All the solutions are explained in a stepwise format helping the students to understand the concepts effortlessly. Wherever necessary, our faculty have provided diagrams in each important concept from the exam perspective, promoting visual learning. Class 12 chemistry ch 6 NCERT solutions are written in crisp and clear as per the requirements of the exams.
8. Why should I download the Vedantu’s NCERT Solutions for Chapter 6 of Class 12 Chemistry PDF?
Students can benefit by downloading these Haloalkanes and Haloarenes class 12 solutions as they are created in an easy-to-understand language. This will help all the students to score good marks in their exams. NCERT Solutions will be helpful for the students not just for their CBSE exams but also for various competitive exams, JEE and NEET exams. Vedantu provides accurate and detailed solutions helping the students to clarify their doubts. It also increases confidence among students to appear for the exam. Haloalkanes and Haloarenes class 12 solutions offer a detailed approach to understanding.
9. What is the most important reaction in haloalkanes and Haloarenes?
The most crucial reaction in haloalkanes and haloarenes is nucleophilic substitution, which is pivotal for altering their chemical structure. Master Haloalkanes and Haloarenes with Class 12 Chemistry Chapter 6 solutions from NCERT.
10. What are the uses of haloalkanes and Haloarenes in daily life?
The most crucial reaction in haloalkanes and haloarenes is nucleophilic substitution, which is pivotal for altering their chemical structure. Class 12 Chemistry Chapter 6 NCERT solutions offer a detailed approach to understanding Haloalkanes and Haloarenes.
11. What are the most important topics of haloalkanes and haloarenes?
Class 12 chemistry ch 6 NCERT solutions Key topics in haloalkanes and haloarenes include nucleophilic substitution reactions, stereochemistry, properties of alkyl and aryl halides, and industrial applications. Master Haloalkanes and Haloarenes Class 12 Chemistry Chapter 6 solutions from NCERT.
12. Why are haloalkanes more reactive than haloarenes?
Haloalkanes exhibit higher reactivity than haloarenes due to the polarisable carbon-halogen bond in sp3 hybridised carbon atoms. Access chemistry class 12 haloalkanes and haloarenes NCERT solutions for comprehensive understanding.
13. Which haloalkanes is most reactive?
Among haloalkanes, primary ones are generally more reactive than secondary or tertiary ones due to lesser steric hindrance. Download NCERT solutions for Class 12 Chemistry Haloalkanes and Haloarenes pdf for in-depth study.
14. What are two uses of haloalkanes?
Due to their low boiling points and chemical stability, haloalkanes are utilised as solvents in organic labs and as refrigerants in air conditioning systems. Enhance your understanding of chemistry class 12 Haloalkanes and Haloarenes NCERT solutions.