Chemical Bonding and Molecular Structure- Class 11 Chemistry Chapter 4 NCERT Solutions



























FAQs on NCERT Solutions for Class 11 Chemistry Chapter 4 - Chemical Bonding and Molecular Structure
1. Give a brief overview of the chapter chemical bonding and molecular structure in NCERT Class 11.
Chemical bonding and molecular structure in NCERT Class 11 deals with the core concepts of molecular structures along with the chemical bonding. This chapter will introduce the concepts of an ionic bond, valence electrons, covalent bonds, etc. Furthermore, students will also learn about the Lewis structure, bond parameters, polar character of covalent bonds, valence bond theory, the geometry of covalent molecules, the covalent character of an ionic bond, etc. You will also read about balanced chemical equations, types of chemical equations, combination reaction, decomposition reaction, displacement reaction, corrosion and rancidity. Further, the students will also be introduced to the concept of hybridization, s, p and d orbitals, VSEPR theory, the molecular orbital theory of homonuclear diatomic molecules hydrogen bond, shapes of simple molecules, etc.
2. What are the topics that are covered in chapter 4 chemical bonding and molecular structure?
Octet Rule
Covalent Bond
Lewis Representation of Simple Molecules (The Lewis Structures)
Formal Charge
Limitations of the Octet Rule
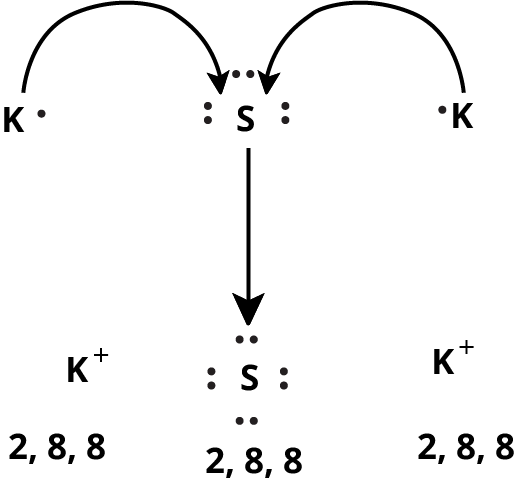
Ionic or Electrovalent Bond
Lattice Enthalpy
Bond Parameters
Bond Length
Bond Angle
Bond Enthalpy
Bond Order
Resonance Structures
Polarity of Bonds
The Valence Shell Electron Pair Repulsion (VSEPR) Theory
Valence Bond Theory
Orbital Overlap Concept
Directional Properties Of Bonds
Overlapping of Atomic Orbitals
Types of Overlapping and Nature of Covalent Bonds
The Strength of Sigma and Pi Bonds
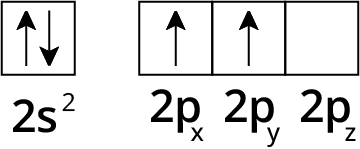
Hybridisation
Types of Hybridisation
Other Examples of Sp3, Sp2 and Sp Hybridisation
The Hybridisation of Elements Involving D Orbitals
Molecular Orbital Theory
Formation of Molecular Orbitals Linear Combination of Atomic Orbitals (LCAO)
Conditions For The Combination of Atomic Orbitals
Types of Molecular Orbitals
Energy Level Diagram For Molecular Orbitals
Electronic Configuration and Molecular Behaviour
Bonding in Some Homonuclear Diatomic Molecules
Hydrogen Bonding
Cause of Formation of Hydrogen Bond
Types of H-bonds.
3. Help me with the process of Chemistry preparation with Vedantu?
Preparing in a step by step and structured manner will help in understanding the concepts in Chemistry easier. First, consider the most scoring and easy concept to score higher marks. The weightage of this topic is also high. You may think of Qualitatives analysis towards the end, as it needs very less time.
chemical bonding class 11 NCERT pdf gives you an in-depth knowledge of the conceptual topics. NCERT Solutions have been drafted as per the latest CBSE Class 11 Science Syllabus. Students will feel that the solutions are in a simple language and can understand the difficult topics easily.
4. What is hybridization?
Redistribution of the energy of orbitals of individual atoms to give orbitals of equivalent energy happens when two atomic orbitals combine together to form hybrid orbital in a molecule. This process is called hybridization. The new orbitals which are formed are known as hybrid orbitals.
Types of Hybridization:
sp Hybridization
sp2 Hybridization
sp3 Hybridization
sp3d Hybridization
sp3d2 Hybridization
5. Give me a summary of NCERT Solutions for Class 11 Chemistry Chapter 4 Chemical Bonding and Molecular Structure.
NCERT Solutions for Class 11 Chemistry Chapter deals primarily with a chemical bond and the attractive force that acts upon it. It talks about atoms, ions, and molecules that all come together to form any given compound. In addition to this, the formation of a chemical bond has been explained via several theories, including the valence shell electron pair repulsion theory, the electronic theory, and the molecular orbital theory.
6. Is it necessary to learn all the questions present in NCERT Solutions for Class 11 Chemistry Chapter 4?
In NCERT Solutions for Class 11 Chemistry Chapter 4, important questions are discussed with their solutions. If you study these questions, you should do fairly well in all your examinations. Thus, although it is not necessary per se to learn all the questions present in chemical bonding NCERT Pdf it will help you in your studies if you do learn them.
7. What is the VSEPR theory?
The Valence Shell Electron Pair Repulsion (VSEPR) theory provides a simple procedure to predict the shapes of covalent molecules. Sidgwick and Powell in 1940 proposed this simple theory based on the repulsive interactions of the electron pairs in the valence shell of the atoms. It was further developed and redefined by Nyholm and Gillespie and stated that the shape of a molecule depends upon the number of valence shell electron pairs around the central atom. Chemical bonding class 11 NCERT pdf provides the best practice to get expertise in the chapter.
8. How can I understand the chapter Chemical Bonding and Molecular structure?
Vedantu provides an excellent repository of study materials for students. In case you are having difficulty understanding any of the chapters in your syllabus, you can check out Vedantu’s online classes or refer to their NCERT solutions. If you read chapters 3 to 4 times and solve questions using NCERT Solutions for Class 11 Chemistry Chapter 4, you will better understand the chapter Chemical Bonding and Molecular Structure. The solutions are free of cost and also available on the Vedantu Mobile app.
9. What are the directional properties of bonds?
Any covalent bond in nature is formed by the overlapping of atomic orbitals. The molecule of hydrogen is formed due to the overlap of 1s-orbitals of two hydrogen atoms. In the case of polyatomic molecules, the geometry of the molecules is also important. The VSEPR theory plays a role in dictating the molecule’s geometry, but the valence bond theory is better suited to explain the directional properties of bonds. Students can also refer to chemistry class 11 chapter 4.
10. What are the most important topics of chemical bonding class 11 NCERT solutions?
Chemical bonding class 11 NCERT solutions covers stoichiometry and chemical reactions, emphasising fundamental concepts and calculations.
11. What are important questions in Chemical Bonding and Molecular Structure class 11?
Chemical bonding Class 11 Solutions revolves around key topics like Lewis structures, molecular geometry, and intermolecular forces.
12. What keeps chemical bonds together?
Electrostatic attraction is the primary force that keeps chemical bonds together, maintaining stability in molecules and compounds. For better understanding Students vedantu also provides chemical bonding class 11 solutions for better exam preparations.
13. What is the most important part in Chemical Bonding and Molecular Structure?
Understanding electron arrangement and the sharing or transfer of electrons is crucial for comprehending the essence of chemical bonding. Students can also refer to chapter 4 chemistry class 11 for better understanding.
14. What are the two main types of chemical bonding?
The two primary types of chemical bonding are ionic bonding, involving transfer of electrons, and covalent bonding, involving sharing of electrons between atoms.